AvaBridge ELISA Kit
본문
| AvaBridge ELISA Kit Complete kit for the systematic 3-D conformational comparability analysis of Avastin biosimilar molecule to Bevacizumab (Avastin trade name).
Bevacizumab is a humanized monoclonal antibody, produced by Genentech, that inhibits vascular endothelial growth factor A (VEGF-A). VEGF-A is a chemical signal that stimulates angiogenesis in a variety of diseases, especially in cancer. Bevacizumab was the first clinically available angiogenesis inhibitor in the United States and was approved by the U.S. Food and Drug Administration (FDA) for certain metastatic cancers. It received its first approval in 2004 for combination use with standardchemotherapy for metastatic colon cancer. This kit will allow for conformational comparison between Avastin Biosimilars and authentic Bevacizumab.
Assay Principle The assay is in a sandwich ELISA format where the plate is coated with a panel of antibodies raised against peptides derived from the full length protein sequence of Bevacizumab. Taken individually, each of these antibodies is strongly antigenic to the peptide sequence that was used in its production. However, when these peptides are incorporated into a full length correctly folded protein, the antigenicity of many of them is masked by the three dimensional structure of the protein and onlya limited number of the antibodies respond. The result is a histogram which can be likened to a ‘fingerprint’ for correctly folded Bevacizumab. For an Avastin Biosimilar, if the protein is correctly folded and glycosylated, the ‘fingerprint’ will match that of Bevacizumab. If it is not correctly folded, previously masked peptide sequences will be exposed and will be recognized by the antibody made to that exposed sequence. In this way, changes in the ‘fingerprint’ generated by the ELISA will point out differencesbetween the Biosimilar and authentic Bevacizumab.
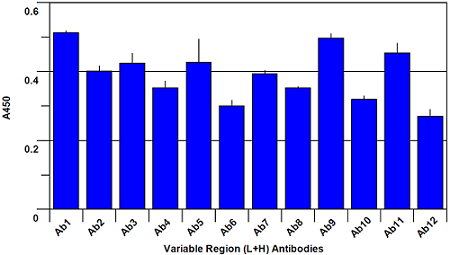
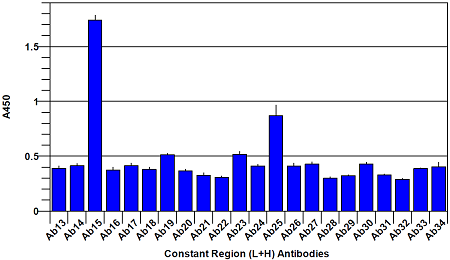
The assay is performed by making a 5 μg/ml solution of Avastin Biosimilar and Bevacizumab reference material respectively, and adding to the 96-well plate. Following a 1 hour incubation to allow capture of the Biosimilar and Bevacizumab reference proteins by the panel of antibodies on the plate, a reporting polyclonal anti-human IgG antibody, conjugated with biotin, is added and incubated for 1 hour to allow it to bind to any captured proteins. After this incubation, the plate is washed anda Streptavidin-HRP (Horse Radish Peroxidase) conjugate is added and incubated for 45 minutes. The Streptavidin-HRP conjugate will be captured by any biotin labeled antibody bound to the plate. Following a wash step to remove unbound conjugate, TMB substrate is added and is converted by the captured HRP to a colored product in proportion to the amount of HRP bound to the plate. After a short incubation to allow color development, the reaction is stopped and the intensity of the generated color is detected in amicrotiter plate reader capable of measuring 450nm wavelength. The color development will be proportional to the captured Biosimilar or Bevacizumab reference protein. A typical ELISA with only the Bevacizumab reference protein is shown in figures 1 and 2 below. Your results may differ from this as your source sample will not be the same one that we used to generate this plot.
Fig. 1. Bevacizumab conformational array ELISA from Variable Region
Fig. 2. Bevacizumab conformational array ELISA from constant region
Product instruction (PDF)
|
Ordering information
| Catalog No. | Target | Size |
| AB000201 | AvaBridge ELISA Kit | Kit |
▣ 관련 페이지 ; Array Bridge
댓글목록
등록된 댓글이 없습니다.