Disease Area, Neuroscience V
Product Name: VX-765 l Caspase 1/Interleukin-converting enzyme inhibitor (#C8976-5)
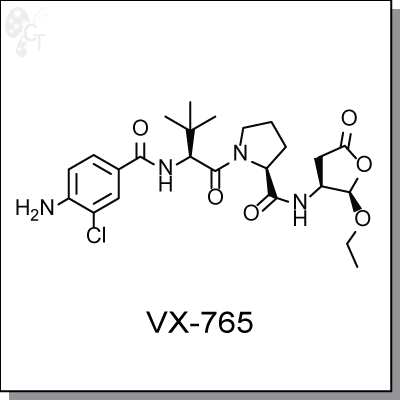
VX-765 is an orally-available prodrug of VRT-043198, an inhibitor of interleukin-converting enzyme /
caspase-1 subfamily caspases. VRT-043198 shows Ki values of 0.8 nM and 100-fold selectivity over other
non-ICE subfamily caspases. VRT-043198 showed no significant activity towards trypsin or cathepsin B. (1)
VRT-043198 inhibits IL-1b release from both PBMCs and whole blood with IC50 values of 0.67 and 1.9 uM,
respectively. Additionally in stimulated PBMCs, VRT-043198 dose-dependently inhibited IL-1b, IL-18, and
IFN-g, without affecting TNF-a release. In a hypoxia-induced apoptosis assay employing the NT2 human
neuroblastoma cell line, VRT-043198 did not alter ischemia-induced apoptosis up to concentrations of 100
uM. (1)
According to Vertex's news release , VX-765 has been shown to inhibit acute seizures in preclinical models
of acute epilepsy and has shown activity in preclinical models of chronic epilepsy that do not respond to
standard anti-epileptic drugs. (2)
|
Details
|
Chemical Formula:
|
|
C24H33CIN4O6
|
|
CAS No.:
|
|
273404-37-8
|
|
Molecular weight:
|
|
509
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
(S)-1-((S)-2-(4-amino-3-chlorobenzamido)-3,3-dimethylbutanoyl)-N-((2R,3S)-2-
ethoxy-5-oxotetrahydrofuran-3-yl)pyrrolidine-2-carboxamide
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Synonyms:
|
|
VX-765, VX765
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
1. Wannamaker et al., (S)-1-((S)-2-{[1-(4-Amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-
butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765),
an Orally Available Selective Interleukin (IL)-Converting Enzyme/ Caspase-1 Inhibitor, Exhibits Potent Anti-
Inflammatory Activities by Inhibiting the Release of IL-1b and IL-18. J. Pharmacol. Exp. Ther. 2007, 321(2),
509-516. Pubmed ID: 17289835
2. http://investors.vrtx.com/releasedetail.cfm?ReleaseID=555967
|
Product Name: YO-01027 (Dibenzazepine) l gamma-secretase inhibitor (#C9601-2s)
.png)
YO-01027 (Dibenzazepine, DBZ) is a dipeptidic g-secretase inhibitor with IC50 of 2.6 and 2.9 nM for APPL
and Notch, respectively. YO-01027 targets the Presenilin fragment. Increasing concentrations of YO-01027
administered to APPl- or Notch-expressing cells leads to the progressive accumulation of APPL C-terminal
fragments and a decrease in NICD production in a dose-dependent manner. [1]
In an in vitro model of human corneal and conjunctival epithelial cell differentation, YO-01027 impaired MUC16
biosynthesis in a concentration-dependent manner. [2]
In oncology models, YO-01027 preferentially inhibited Notch and significantly decreased MCF7 tumors and
increased latency compared with control mice. [3]
|
Details
|
Chemical Formula:
|
|
C26H23F2N3O3
|
|
CAS No.:
|
|
209984-56-5
|
|
Molecular weight:
|
|
463.48
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
7-(S)-[N'(3,5-difluorophenylacetyl)-L-alaninyl]amino-5-methyl-5,7-dihydro-6H-
dibenz[b,d]azepin-6-one
|
|
Solubility:
|
|
Up to 100mM in DMSO
|
|
Synonyms:
|
|
YO-01027, YO01027, Dibenzazepine, Deshydroxy LY 411575
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Groth et al., Pharmacological Analysis of Drosophila melanogaster g-Secretase with Respect to Differential
Proteolysis of Notch and APP. Mol. Pharmacol. 2010, 77(4), 567-574. Pubmed ID: 20064975
2. Xiong et al., Notch Signaling Modulates MUC16 Biosynthesis in an In Vitro Model of Human Corneal and
Conjunctival Epithelial Cell Differentiation. Invest. Ophthalmol. Vis. Sci. 2011, 52(8), 5641-5646. Pubmed
ID: 21508102
3. Harrison et al., Regulation of Breast Cancer Stem Cell Activity by Signaling through the Notch4 Receptor. Cancer Res., 2010, 70, 709-718. Pubmed ID: 20068161
|
|

.png)