Disease Area, Neuroscience IV
Product Name: Prucalopride l 5-HT4 receptor agonist (#C7782-5)
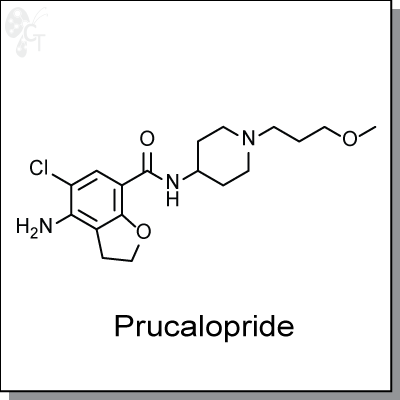
Prucalopride is an orally-available, dibenzofuran-based, enterokinetic agonist of the human serotonin 5-HT4a
and 5-HT4b receptor isoforms with Ki estimates of 3 nM and 8 nM, respectively. It has modest selectivity
over the human D4 receptor (2.3 uM), mouse 5-HT3 receptor (3.7 uM) and human s1 receptor (3.7 uM). (1, 2)
Initial tolerability studies show that prucalopride does not encounter cardiotoxicity issues to the extent seen
in other drugs of this class. [3]
|
Details
|
Chemical Formula:
|
|
C18H26ClN3O3
|
|
CAS No.:
|
|
179474-81-8
|
|
Molecular weight:
|
|
367.87
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
7-Benzofurancarboxamide, 4-amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-
4-piperidinyl]
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
0
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
1. Briejer et al., The in vitro pharmacological profile of prucalopride, a novel enterokinetic compound, Eur. J.
Pharmcol. 2001, 423, 71-83. Pubmed ID: 11438309
2. Frampton et al., Prucalopride: ADIS Drug Profile. Drugs, 2009, 69(17), 2453-2476. Pubmed ID: 19911858
3. Quigley et al., Prucalopride: safety, efficacy and potential applications. Ther. Adv. Gastroenterol. 2012,
5(1), 23-30.
|
Product Name: RO4929097 l γ-secretase inhibitor(#C7649-2s)
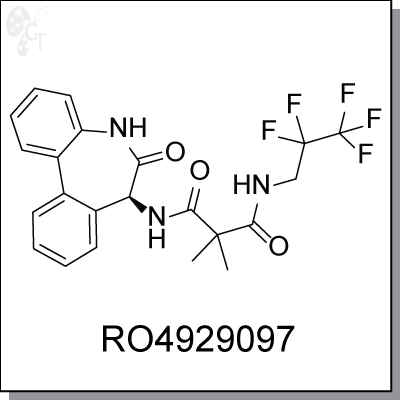
RO4929097 is a potent and selective small molecule inhibitor of γ-secretase (IC50=4nM), producing inhibitory
activity of Notch signaling in tumor cells. It inhibits Notch processing in tumor cells as measured by the
reduction of intracellular Notch expression by Western blot. This leads to reduced expression of the Notch
transcriptional target gene Hes1. RO4929097 does not block tumor cell proliferation or induce apoptosis but
instead produces a less transformed, flattened, slower-growing phenotype. Current it is in phase I study in
patients with advanced solid tumors.
Product Citation
NOTE: Under licensing agreement with Hoffmann-La Roche Ltd, this product is to be used
for in vitro study only.
|
Details
|
Chemical Formula:
|
|
C22H20F5N3O3
|
|
CAS No.:
|
|
847925-91-1
|
|
Molecular weight:
|
|
469.40
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical name:
|
|
2,2-dimethyl-N-((S)-6-oxo-6,7-dihydro-5H-dibenzo[b, d]azepin-7-yl)-N′-
(2,2,3,3,3-pentafluoro-propyl)-malonamide
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
Reference
1. Luistro L, He W et al. Preclinical profile of a potent gamma-secretase inhibitor targeting notch signaling
with in vivo efficacy and pharmacodynamic properties. Cancer Res. 2009;69(19):7672-80.
|
Product Name: SPM-927 (Lacosamide) (#C7927-5)
.png)
Lacosamide is a small peptide-based agent with a dual mode of action for anticonvulsive and antiepileptic
treatment. Lacosamide binds weakly (25-50% inhibition of radioligand binding) to the sodium channel at
batrachotoxin site 2. It selectively enhances sodium channel slow inactivation with no effects on fast
inactivation. This slow mode of action can lead to normalization of activation thresholds and a reduced
pathophysiological hyperresponsiveness. Lacosamide was also shown to be a binding partner to collapsin-
response mediator protein 2 (CRMP-2 or DRP-2) with a binding affinity of 5 uM. [1]
Extensive binding studies have shown that Lacosamide and its metabolites do not significantly bind to any of
the known binding sites of other anticonvulsant or analgesic agents. Furthermore, it experiences no uptake or
metabolism in major neurotransmitters. [2]
Lacosamide is also being studied in animal models of pain and inflammation.
|
Details
|
Chemical Formula:
|
|
C13H18N2O3
|
|
CAS No.:
|
|
175481-36-4
|
|
Molecular weight:
|
|
250.29
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
(2R)-2-(Acetylamino)-3-methoxy-N-(phenylmethyl)propanamide
|
|
Solubility:
|
|
Up to 22 mM in DMSO
|
|
Synonyms:
|
|
SPM-927, SPM927, Lacosamide, Erlosamide, Harkoseride, Vimpat
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Beyreuther et al., Lacosamide: a review of preclinical properties. CNS Drug, 2007, 13(1), 21-42.
Pubmed ID:17461888
2. Kellinghaus et al., Lacosamide as treatment for partial epilepsy: mechanisms of action, pharmacology,
effects, and safety. Therapeut. Clin. Risk Mgmt. 2009, 5, 757-766. Pubmed ID: 19816574
|
Product Name: SYN115 | Adenosine A(2a) receptor antagonist (#C7911-5)
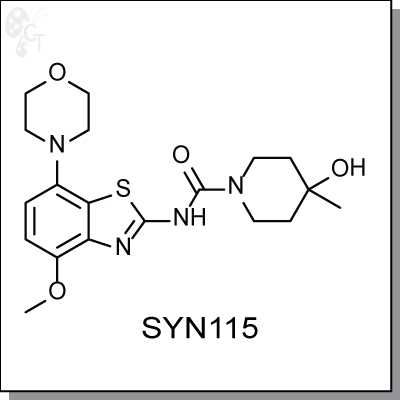
SYN115 (Tozadenant) is a orally-available, benzothiazole-based, antagonist of the Adenosine A2A receptor
for the treatment of Parkinson's disease and other CNS-related conditions. SYN115 is being pursued as a
monotherapy as well as in combination with L-dopamine and is being studied for its potential neuroprotective
effects. [1]
Animal studies with SYN115 support beliefs that A2A antagonists reduce Parkinson's symptoms by reducing
the inhibitory output of the basal ganglia indirect pathway. Using Cerebral Blood Flow (CBF) as a
pharmacodynamic parameter, studies have shown that SYN115 induces a significant decrease in thalamic
CBF, consistent with the deactivation of the indirect pathway. [2]
|
Details
|
Chemical Formula:
|
|
C19H26N4O4S
|
|
CAS No.:
|
|
870070-55-6
|
|
Molecular weight:
|
|
406.5
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
4-Hydroxy-N-[4-methoxy-7-(4-morpholinyl)-2-benzothiazolyl]-4-methyl-1-
piperidinecarboxamide
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Synonyms:
|
|
SYN115, SYN-115
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Biotie Therapies website (2013-04-24)
2. Black et al., Quantification of Indirect Pathway Inhibition by the Adenosine A2a Antagonist SYN115 in
Parkinson Disease. Black et al., J. Neurosci. 2010, 30(48), 16284-16292. Pubmed ID: 21123574
|
|


.png)
