Disease Area, Neuroscience III
Product Name: LY2886721 l BACE inhibitor (#C5288-5)
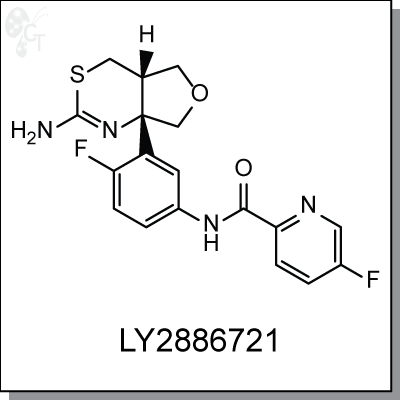
LY2886721 is an orally-available, furothiazine-based inhibitor of b-amyloid protein cleaving enzyme (BACE)
for the treatment of Alzheimer's disease. [1] It inhibits recombinant hBACE1 with an IC50 of 20 nM. [2] In
cellular assays, LY2886721 inhibits Abeta with an IC50 range of 10-19 nM. Oral administration of LY2886721 to
PDAPP mice produced dose-dependent reductions in brain Abeta, C99, and sAPPbeta. [2]
|
Details
|
Chemical Formula:
|
|
C18H16F2N4O2S
|
|
CAS No.:
|
|
1262036-50-9
|
|
Molecular weight:
|
|
390.41
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
N-(3-((4aS,7aS)-2-amino-4a,5,7,7a-tetrahydro-4H-furo[3,4-d][1,3]thiazin-7a-yl)-4-
fluorophenyl)-5-fluoropicolinamide
|
|
Solubility:
|
|
Up to 20 mM in DMSO
|
|
Synonyms:
|
|
LY-2886721, LY2886721, LY2886721
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
1. Alzheimer's Disease Research Unit Studies synopsis (April 1, 2012)
2. Alzheimer Assocation International Conference, 2012, Oral Sessions (O1-06-03): "Preclinical
Characterization of LY2886721: A BACE1 inhibitor in clinical developmetn for early Alzheimer's disease"
|
Product Name: P7C3 l Neurogenic & neuroprotective agent (#C7723-10)
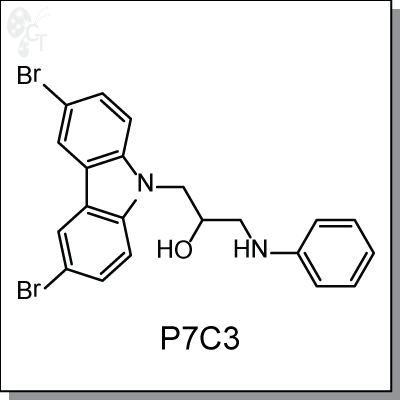
P7C3 is a proneurogenic, neuroprotective small molecule with favorable pharmacological properties
discovered from an in vivo screen. In vivo studies gave evidence that P7C3 exerts its proneurogenic activity
by protecting newborn neurons from apoptosis. Prolonged administration of P7C3 to npas3-/- mice corrected
their deficits by normalizing levels of apoptosis of newborn hippocampal neurons. Prolonged administration
of P7C3 to aged rats also enhanced neurogenesis in the dentate gyrus, impeded neuron death, and
preserved cognitive capacity as a function of terminal aging.
|
Details
|
Chemical Formula:
|
|
C21H18Br2N2O C21H18Br2N2O
|
|
CAS No.:
|
|
301353-96-8
|
|
Molecular weight:
|
|
474.19
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical name:
|
|
1-(3,6-dibromo-9H-carbazol-9-yl)-3-(phenylamino)propan-2-ol
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Andrew A Pieper, et al. Discovery of a proneurogenic, neuroprotective chemical. Cell (2010), 142(1), 39-51.
2. Karen S MacMillan, et al. Development of Proneurogenic, Neuroprotective Small Molecules. Journal of the
American Chemical Society (2011), 133(5), 1428-1437.
|
Product Name: PF-3845 | FAAH inhibitor (#C7384-5)
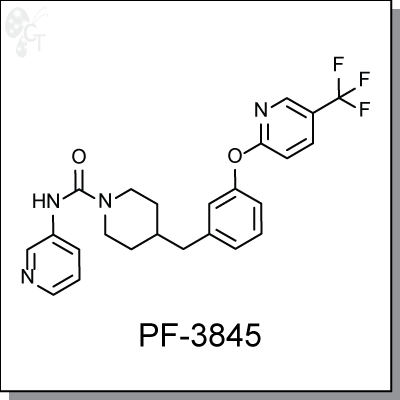
PF-3845 is an orally-available, covalent and irreversible inhibitor of fatty acid amide hydrolase (FAAH) for the
treatment of inflammation and pain, with an IC50 of 7.2 nM. [1] Mechanistic studies show that PF-3845 is a
time-dependent inhibitor that carbamylates FAAH's catalytic serine nucleophile and raises anandamide
levels in the brain for up to 24h. [2, 3]
Oral administration of PF-3845 produces antinociceptive effects in both inflammatory and noninflammatory
pain models in rats with an MED 0.1 mg/kg. [2] Furthermore, oral administration of PF-3845 at 0.1 mg/kg
results in efficacy comparable to that of naproxen at 10 mg/kg in a rat inflammatory pain model. [1]
Regarding the encannabinoid system, PF-3845 has been shown to be an effective treatment (i.p.) for the
blockade of neuronal FAAH to reverse allodynia through the activation of both cannabinoid receptors, without
the psychomimetic side effects associated with THC. [4]
|
Details
|
Chemical Formula:
|
|
C24H23F3N4O2
|
|
CAS No.:
|
|
1196109-52-0
|
|
Molecular weight:
|
|
456.46
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
4-(3-(5-(trifluoromethyl)pyridin-2-yloxy)benzyl)-N-(pyridin-3-yl)piperidine-1-
carboxamide
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
PF-3845, PF 3845, PF3845
|
|
Storage:
|
|
For longer shelf life, store solid powder or DMSO solution at -20oC
|
References
1. Johnson et al., Discovery of PF-04457845: A Highly Potent, Orally Bioavailable, and Selective Urea FAAH
Inhibitor. ACS Med. Chem. Lett. 2011, 2, 91-96. Pubmed ID: 21666860
2. Ahn et al., Mechanistic and pharmacological characterization of PF-04457845: a highly potent and selective
fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J. Pharmcol.
Exp. Ther. 2011, 338 (1), 114-124. Pubmed ID: 21505060
3. Ahn et al., Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory
pain. Chem. Biol. 2009, 16(4), 411-420. Pubmed ID: 19389627
4. Booker et al., The fatty acid amide hydrolase (FAAH) inhibitor PF-3845 acts in the nervous system to
reverse LPS-induced tactile allodynia in mice. Br. J. Pharmacol. 2012, 165(8), 2485-2496. Pubmed ID:
21505060
|
Product Name: PNU-282987 | nAChR agonist (#C7282-5)
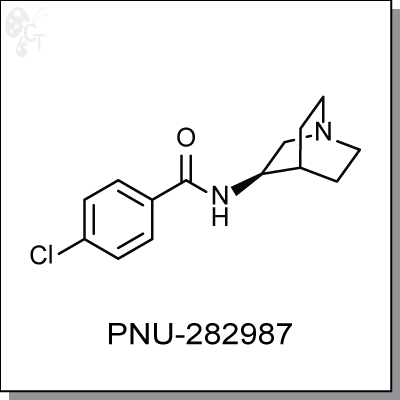
PNU-282987 is an α7 nAChR agonist with an EC50 of 154 nM against the a7-5HT3 chimera. Up to
concentrations of 100 uM, PNU-282987 displayed no agonist activity and negligible antagonistic activity (60
uM) toward the neuromuscular junction form of the receptor and the ganglionic nAChR (a3b4). PNU-282987 is
a functional antagonist of the 5-HT3 receptor at an IC50 of 4.5 uM. (1)
Cotreatment of PC12 cells with nAChR modulator PNU120596 and PNU-282987 significantly induces ERK1/2
phosphorylation. (2) In a chloral hydrate-anesthetized rat model, PNU-282987 was shown to restore
amphetamine-induced sensory gating deficit and may have implications in the treatment of schizophrenia.
(3)
|
Details
|
Chemical Formula:
|
|
C14H17ClN2O
|
|
CAS No.:
|
|
123464-89-1
|
|
Molecular weight:
|
|
264.75
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
N-(3R)-1-Azabicyclo[2.2.2]oct-3-yl-4-chlorobenzamide
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
PNU-282987, PNU 282987, PNU282987
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Bodnar et al., Discovery and Structure-Activity Relationship of Quinuclidine Benzamides as Agonists of a7
Nicotinic Acetylcholine Receptors. J. Med. Chem. 2005, 48, 905-908. Pubmed ID: 15715459
2. El Kouhen et al., Pharmacology of a7 nicotinic acetylcholine receptor mediated extracellular signal-
regulated kinase signalling in PC12 cells. Br. J. Pharmacology, 2009, 156, 638-648. Pubmed ID: 19226255
3. Hajos et al., The Selective a7 Nicotinic Acetylcholine Receptor Agonist PNU-282987 [N-[(3R)-1-Azabicyclo
[2.2.2]oct-3-yl]-4-chlorobenzamide Hydrochloride] Enhances GABAergic Synaptic Activity in Brain Slices
and Restores Auditory Gating Deficits in Anesthetized Rats. J. Pharmcol. Exp. Ther. 2005, 312(3), 1213-
1222. Pubmed ID: 15523001
|
|



