Disease Area, Neuroscience II
Product Name: BMS-708163 (Avagacestat) l γ-secretase inhibitor (#C3670-5)
.png)
BMS-708163 (Avagacestat) is a potent and selective inhibitor for gamma-secretase, especially at inhibiting
cleavage of amyloid precursor protein (APP) to form amyloid-ß (Aß)(1-40) (IC50=0.3nM) and Aß(1-42)
(0.27nM). BMS-708163 is Notch sparing, with more than 100 folds more selective for APP than for Notch
protein (IC50 of 58nM).
BMS-708163 is developed for treating Alzheimers disease (AD). Oral administration of BMS-708163
significantly reduced Aß(1-40) levels for sustained periods in brain, plasma, and cerebrospinal fluid in rats
and dogs [1]. The results of Phase II clinical trial in mild-to-moderate AD patients demonstrated acceptable
tolerability at a single-dose range of 0.3 to 800 mg with a biphasic effect on plasma Aß(1-40) [2].
|
Details
|
Chemical Formula:
|
|
C20H17ClF4N4O4S
|
|
CAS No.:
|
|
1146699-66-2
|
|
Molecular weight:
|
|
520.88
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
(2R)-2-(N-(2-fluoro-4-(1,2,4-oxadiazol-3-yl)benzyl)-4-chlorophenylsulfonamido)-
5,5,5-trifluoropentanamide
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Synonyms:
|
|
BMS-708163, BMS 708163, BMS708163, Avagacestat
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
1. Gillman, KW., et al. Discovery and Evaluation of BMS-708163, a Potent, Selective and Orally Bioavailable ?-
Secretase Inhibitor. ACS Med. Chem. Lett., 2010, 1 (3): 120124
2. Tong G., et al., Multicenter, randomized, double-blind, placebo-controlled, single-ascending dose study
of the oral ?-secretase inhibitor BMS-708163 (Avagacestat): tolerability profile, pharmacokinetic parameters,
and pharmacodynamic markers. Clin Ther. 2012 . 34(3):654-67. Pubmed ID: 22381714
|
Product Name: J147 l Neuroprotective compound (#C5147-2s)
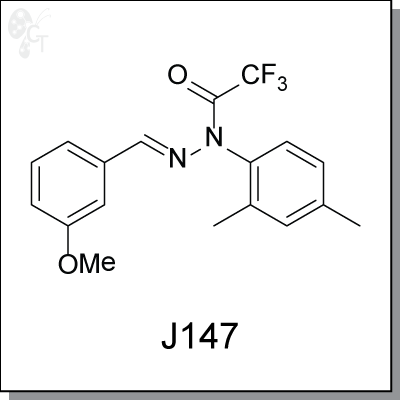
J147 was discovered based upon efficacy in multiple cell culture models of age-associated pathologies
rather than exclusively amyloid metabolism. It is an exceptionally potent, orally active and broadly
neuroprotective compound has the ability to enhance memory in normal animals as well as to prevent
memory deficits in Alzheimer’s disease (AD) transgenic mice. The neurotrophic and memory-enhancing
activities of J147 are associated with an increase in brain derived neurotrophic factor (BDNF) levels and the
expression of BDNF responsive proteins, the enhancement of LTP, the preservation of synaptic protein, the
reduction of amyloid plaques, etc. These pleiotrophic effects of a single molecule suggest that J147 has
potential for the treatment of AD.
|
Details
|
Chemical Formula:
|
|
C18H17F3N2O2
|
|
CAS No.:
|
|
1146963-51-0
|
|
Molecular weight:
|
|
350.33
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Yellow solid
|
|
Chemical name:
|
|
(E)-N-(2,4-dimethylphenyl)-2,2,2-trifluoro-N'-(3-methoxybenzylidene)
acetohydrazide
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Synonyms:
|
|
ABR-215062, ABR 215062, ABR215062, Laquinimod
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
Reference
1. Qi Chen, et al. A novel neurotrophic drug for cognitive enhancement and Alzheimer's disease. PLoS One
(2011), 6(12), e27865.
|
Product Name: LDN-57444 | UCH-L1 inhibitor (#C5574-5)
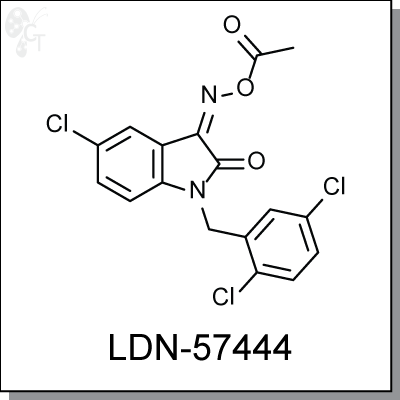
LDN-57444 is a specific inhibitor (Ki = 0.4 ?M) against Ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), a
member of deubiquitinating enzymes (DUBs). LDN-57444 increased proliferation of SH-SY5Y cells, a UCH-
L1-expressing neuroblastoma line. [1]
As expression of UCH-L1 is highly specific to neurons and to cells of the diffuse neuroendocrine system and
their tumors, LDN-57444 is a useful tool for studying the roles of UCH-L1 in Alzheimers disease,
Parkinsons disease, and other neurological disorders. [2,3]
|
Details
|
Chemical Formula:
|
|
C17H11Cl3N2O3
|
|
CAS No.:
|
|
668467-91-2
|
|
Molecular weight:
|
|
397.64
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Yellow
|
|
Chemical name:
|
|
1H-Indole-2,3-dione, 5-chloro-1-[(2,5-dichlorophenyl)methyl]-, 3-(O-acetyloxime)
|
|
Solubility:
|
|
Up to 25 mM in DMSO
|
|
Synonyms:
|
|
LDN-57444, LDN57444
|
|
Storage:
|
|
For longer shelf life, store solid powder or DMSO solution at -20oC
|
References
1. Liu Y, et al. Discovery of inhibitors that elucidate the role of UCH-L1 activity in the H1299 lung cancer cell
line. Chem Biol. 2003; 10(9):837-46. Pubmed ID: 14522054
2. Cartier AE, et al. Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1. J Neurosci. 2009; 29
(24):7857-68. Pubmed ID: 19535597
3. Zhang M, et al. Control of BACE1 degradation and APP processing by ubiquitin carboxyl-terminal
hydrolase L1. J Neurochem. 2012; 120(6):1129-38. Pubmed ID: 22212137
|
Product Name: LY2811376 | BACE inhibitor (#C5281-5)
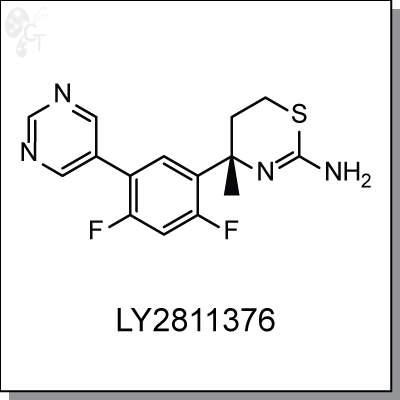
LY-2811376 is a orally-available, non-peptidic inhibitor of hBACE1 with an IC50 of 239 nM. Selectivity of LY-
2811376 for BACE1 over BACE2 was found to be 10-fold, and 50-fold or greater over related aspartyl proteases
cathepsin D, pepsin, or renin. [1] In APP-overexpressing human embryonic kidney cell lines, LY-2811376
treatment decreased Ab secretion in a concentration-dependent fashion, with an EC50 of ~300 nM. In primary
neuronal cultures of PDAPP transgenic mouse, Ab secretion was measured at an EC50 of ~100 nM.
In vivo, LY2811376 inhibits BACE1 activity by concomitantly reducing C99 and sAPPb, the two primary APP
cleavage products generated by BACE1.
|
Details
|
Chemical Formula:
|
|
C15H14F2N4S
|
|
CAS No.:
|
|
1194044-20-6
|
|
Molecular weight:
|
|
320.36
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
(S)-4-(2,4-difluoro-5-(pyrimidin-5-yl)phenyl)-4-methyl-5,6-dihydro-4H-1,3-thiazin-
2-amine
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Synonyms:
|
|
LY-2811376, LY 2811376, LY2811376
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
Reference
1. May et al., Robust central reduction of amyloid-? in humans with an orally available, non-peptidic ?-
secretase inhibitor. J. Neurosci., 2011, 31(46), 16507-16516. Pubmed ID: 22090477
|
|
.png)


