Disease Area, Neuroscience I
Product Name: 7,8-Dihydroxyflavone (#C7181-10)
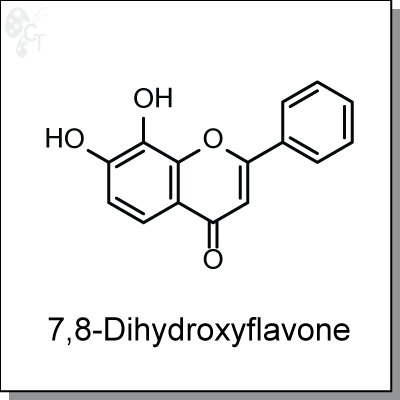
7,8-Dihydroxyflavone binds to the extracellular domain of tyrosine kinase receptor B (Kd = 320 nM) and
activate the receptor activity. It inhibits glutamate-triggered apoptosis in hippocampal neurons in vitro and in
vivo. Administration of 7,8-dihydroxyflavone to mice activated TrkB in the brain, inhibited kainic acid-induced
toxicity, decreased infarct volumes in stroke in a TrkB-dependent manner, and was neuroprotective in an
animal model of Parkinson disease. It also reverses memory deficits and BACE1 elevation in a mouse model
of Alzheimer's Disease.
|
Details
|
Chemical Formula:
|
|
C15H10O4
|
|
CAS No.:
|
|
38183-03-8
|
|
Molecular weight:
|
|
254.24
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical name:
|
|
7,8-dihydroxy-2-phenyl-4H-chromen-4-one
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
1. Sung-Wuk Jang et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone.
Proc.Natl.Acad.Sci.USA. 2010; 107 268.
2. Latha Devi et al. 7,8-Dihydroxyflavone, a Small-Molecule TrkB Agonist, Reverses Memory Deficits and
BACE1 Elevation in a Mouse Model of Alzheimer's Disease. Neuropsychopharmacology advance online
publication 7 September 2011.
3. Zhang R et al. Preventive effect of 7,8-dihydroxyflavone against oxidative stress induced genotoxicity. Biol
Pharm Bull. 2009;32(2):166-71
4. Raul Andero et al. Effect of 7,8-Dihydroxyflavone, a Small-Molecule TrkB Agonist, on Emotional Learning.
Am J Psychiatry 2011; 168:163-172.
|
Product Name: ABR-215062 (Laquinimod) l Immunomodulator (#C2215-2s)
.png)
Laquinimod is an orally-available. quinolinone-based, broad spectrum immunomodulator with anti-
inflammatory activity, most likely by effecting a T-helper cell 1 to 2 cytokine shift. [1] Additionally it reduces
antigen presentation and the effect on migration of T-cells. Laquinimod is also believed to provide a
neuroprotective effect and may have application to relapsing-remitting multiple sclerosis (RRMS). [2]
In animal models for multiple sclerosis, Laquinimod reversed established relapsing remitting experimental
autoimmune encephalomyelitis (RR EAE) and was associated with reduced CNS inflammation, decreased
Th1 and Th17 responses, and an increase in regulatory T cells. [3]
|
Details
|
Chemical Formula:
|
|
C19H17ClN2O3
|
|
CAS No.:
|
|
248281-84-7
|
|
Molecular weight:
|
|
356.8
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical name:
|
|
5-chloro-N-ethyl-4-hydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-
carboxamide
|
|
Solubility:
|
|
Up to 22 mM in DMSO
|
|
Synonyms:
|
|
ABR-215062, ABR 215062, ABR215062, Laquinimod
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. "Laquinimod for relapsing-remitting multiple sclerosis" National Horizon Scaning Centre technology
summary, Aug. 2011.
2. West et al., Profile of oral laquinimod and its potential in the treatment of multiple sclerosis. Degenerative
Neurological Muscular Disease 2011, 1, 25-32.
3. Schulze-Topphoff et al., Laquinimod, a quinoline-3-carboxamide, induces type II myeloid cells that
modulate central nervous system autoimmunity. PLoS ONE 2012, 7(3), e33797. Pubmed ID: 22479444
|
Product Name: AG-014699 (PF-01367338, Rucaparib) |PARP 1/2 inhibitor (#C2401-5)
.png)
AG-014699 (Rucaparib) is an intraveneously-administered, azepinone-indole-based inhibitor of PARP 1 and 2
(Ki = 1.4 nM) [1] AG-014699 induces selective cytotoxicity in tumor cells defective in homologous
recombination repair (HRR) through BRCA1 and 2 mutation and non-BRCA mutated HRR defects. It is a potent
chemosensitizer of temozolomide (3 to 10 fold) and topotecan (1.5 to 2.3 fold) antitumor activity in
neuroblastoma cells. [2, 3]
|
Details
|
Chemical Formula:
|
|
C19H18FN3O.H3PO4
|
|
CAS No.:
|
|
459868-92-9
|
|
Molecular weight:
|
|
421.36
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Yellow
|
|
Chemical name:
|
|
8-Fluoro-2-(4-methylaminomethyl-phenyl)-1,3,4,5-tetrahydro-azepino[5,4,3-cd]
indol-6-one phosphate
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
AG-014699, AG014699, AG-14699, AG-014447, PF-01367338, PF01367338, Rucaparib
phosphate, Rucaparib
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. "Phase II trial of the Poly(ADP-ribose) polymerase (PARP) inhibitor AG-014699 in BRCA1 and 2 mutated
advanced ovarian and breast cancer" Cancer Research UK / Newcastle Univ. Abstract 3104
2. Thomas et al., Preclinical selection of a novel poly(ADP-ribose) polymerase inhibitor for clinical trial. Mol.
Cancer. Ther. 2007, 6(3), 945-956. Pubmed ID: 17363489
3. Daniel et al., Inhibition of poly(ADP-ribose) polymerase-1 enhances temozolomide and topotecan activity
against childhood neuroblastoma. Clin. Cancer Res. 2009, 15(4), 1241-1249. Pubmed ID: 19174487
|
Product Name: AGI-5198 (IDH-C35) | IDH1 inhibitor (#C2519-5)
.png)
AGI-5198 (IDH-C35) is a potent and selective isocitrate dehydrogenase 1 (IDH1) inhibitor specifically against
R132H/C mutants (mIDH1) with IC50 at 100 nM range. AGI-5198 does not inhibit wild-type IDH1 or any of the
examined IDH2 isoforms (IC50 > 100 µM).[1] AGI-5198 inhibits R-2-hydroxyglutarate (R-2HG) production by
mIDH1. AGI-5198 also induces demethylation of histone H3K9me3 and expression of genes associated with
gliogenic differentiation.
In both in vitro and in vivo studies, AGI-5198 inhibited the growth of glioma cells carrying mutated but not wild
type IDH1, yet with no appreciable changes in genome-wide DNA methylation. These data suggest that
mIDH1 may promote glioma growth through mechanisms beyond its well-characterized epigenetic effects.
AGI-5198 could serve as a very useful chemical tool to probe the mechanism and treatment of mIDH1-
carrying tumors
|
Details
|
Chemical Formula:
|
|
C27H31FN4O2
|
|
CAS No.:
|
|
1355326-35-0
|
|
Molecular weight:
|
|
462.56
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
N-cyclohexyl-2-(N-(3-fluorophenyl)-2-(2-methyl-1H-imidazol-1-yl)acetamido)-2-
(o-tolyl)acetamide
|
|
Solubility:
|
|
Up to 25 mM in DMSO
|
|
Synonyms:
|
|
AGI-5198, AGI5198, IDH-C35
|
|
Storage:
|
|
For longer shelf life, store solid powder or DMSO solution at -20oC
|
Reference
1. Rohle D, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells.
Science. 2013; 340(6132):626-30. Pubmed ID: 23558169
|
|

.png)
.png)
.png)