ExoTEST, ELISA exosome quantification kit
본문
|
ExoTESTTM, ELISA exosome quantification kit Double sandwich ELISA assay for exosome immunocapture and quantification from human biofluids or cell culture media
ExoTESTTM, Ready to use kit for exosome capture and quantification ExoTEST™ is a patented double sandwich ELISA assay for quantitative and qualitative analysis of exosomes. In particular ExoTEST™ is a successful platform for exosomes quantification and characterization from small amount of human biological fluids or cell media and it may be exploited to identify exosomes released by cancer cells in the plasma and urine of tumor patients in various disease conditions.
I
ExoTEST™ consists in ELISA plates pre-coated with proprietary pan-exosome antibodies enabling specific capture of exosomes from different biological samples, including cell culture supernatants and human biological fluids. Quantification and characterization of exosomal proteins is subsequently performed using appropriate detection antibodies against exosome associated antigens that can be for either genericor cell/tissue-specific exosomes. Lyophilized Exosome Standards, charcterized for protein content and particle number (NTA) allow the quantification of unknown sample by a standard calibration curve.
Features • Ready to use • No initial exosome purification required • User friendly and suitable for multiple marker analyses • Available in TEST format (limited to 3 ELISA strips, 24 wells)
Applications • Exosome capture and quantification from human biofluids and cell culture media • Exosome comprehensive profiling • Pre-clinical research on non-invasive biomarkers for detection and monitoring of a number of pathological conditions (inflammation, cancer, neurodegeneration, etc.)
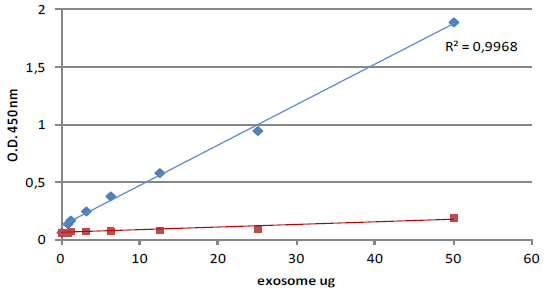
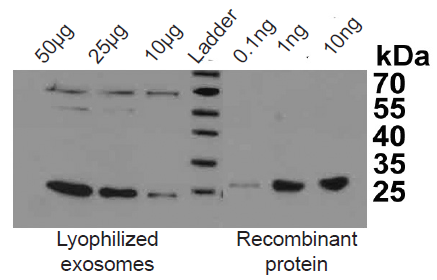
ExoTESTTMshows high sensitivity in detecting low exosome amount, high affinity and low background for exosome binding and quantification. The sensitivity of the ExoTEST™ was compared to Western blot. The sensitivity of the ExoTEST™ is higher than Western blotting as reported in Figures 1 and 2 showing that 10 μg of lyophilized exosomes are equivalent to 0.1 ng of recombinant exosomal protein. Since the standard curve’s lower concentration is 0.39 μg of lyophilized exosomes (Fig. 1), the sensitivity of our test is around 39 pg of protein equivalent.In addition, immunoplates used for exosome capture shows a low background which makes it a reliable, efficient and accurate test (Fig. 1).
Fig. 1. CD9 titration (blue line) of plasma healthy donor exosome standards (HBM-PEP100) and comparison with observed background (red line, only secondary antibody).
Fig. 2. CD9 exosome marker detection by Western Blotting on lyophilized exosomes from human plasma (HBM-PEP100) and recombinant CD9 protein
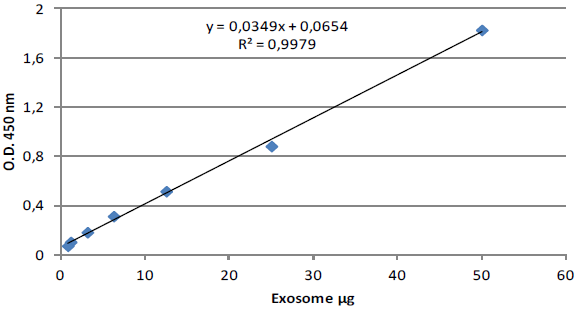
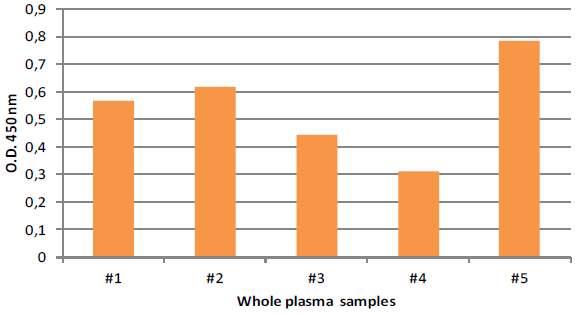
ExoTEST™ is a sensitive method for exosome quantification in human biofluids ExoTEST™ enables robust and precise quantification of exosomes from human biofluids, exhibiting consistency among individual samples and different experiments. Standard exosome preparations are provided in the kit to design standard curves and for assay calibration. We report an example of exosome quantification performed in 5 unknown plasma samples from healthy donors using the ExoTEST™ Ready to use kit forOverall Exosome quantification from human plasma (HBM-RTK-POF/TP). Following Lyophilized Standards and unknown samples binding onto the ELISA plate, test is run in according to the kit protocol and exosome detection is performed with anti-CD9 antibody (HBM).
Fig. 3. Standard curve obtained with Lyophilized Exosome Standards from human plasma healthy donors (HBM-PEP100) with anti-CD9 antibody.
Fig. 4. CD9 titration of exosomes in 5 different whole plasma from healthy donor samples.
Table 1. Exosome quantification is performed calculating the quantity of exosomes (expressed in μg) into the 5 unknown samples through the equation of the standard curve (Fig 3). The particles number contained in 100 μl of plasma is calculated from quantity of exosomes (expressed in μg) in according to the particles concentration (number of particles/ml) indicated in the label of the Lyophilized Exosome Standards(HBM- PEP100, NTA: 3x10^11 particles/ml)
HBM provides different types of ExoTESTTMkit for overall or specific exosome subpopulation capture and quantification HBM provides several ExoTEST™ kits for quantification of overall or specific exosome populations from human biofluids (plasma, urine, serum) and from cell culture supernatants. ExoTEST™ are also available with specific immunoplates for colorimetric (transparent), luminometric (white) or fluorimetric (black) readings. Customized ExoTEST™ kits can be provided for special research needs or for OEM productions.
Ordering informations
- ExoTEST™ kits for overall exosome immunocapture and quantification from biological fluids
- ExoTEST™ Ready to use kit for tumor-derived exosome enrichment and quantification from biological fluids
|
▣ 관련 페이지 ; HansaBioMed
- 이전글Exo-FACS, Ready to use kit for exosome FACS analysis 16.01.05
- 다음글Anti-BRAF (V600E), rabbit mAB_updated 15.12.23
댓글목록
등록된 댓글이 없습니다.