Disease Area, Musculoskeletal
Product Name: INCB018424 l JAK1/2 inhibitor (#C4622-2s)
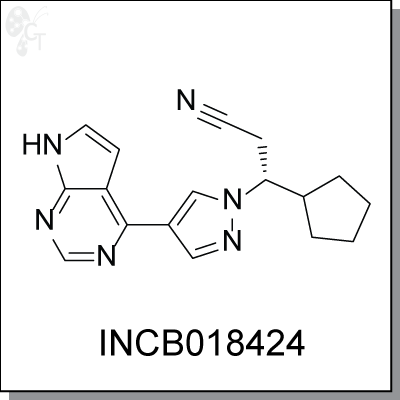
INCB018424 is an orally bioavailable, potent and selective inhibitor of JAK 1 and JAK2, currently in clinical
trials to treat myelofibrosis. In enzyme assays it inhibited JAK1 (IC50=3.2 nM) and JAK2 (4.1 nM), and had
selectivity >100 fold for a wide range of other kinases, including JAK3 (428 nM). It acts by blocking the
JAK/STAT pathway, and has IC50 of 280nM in cytokine-stimulated whole blood assays.
|
Details
|
Chemical Formula:
|
|
C17H18N6
|
|
CAS No.:
|
|
941676-49-5
|
|
Molecular weight:
|
|
306.37
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical name:
|
|
(R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-
cyclopentylpropanenitrile
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
1. A Quintas-Cardama et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424:
therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 2010, 115(15), 3109-3117.
2. S Verstovsek et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis.
N. Engl. J. Med. 2010, 363(12), 1117-1127.
|
Product Name: Strontium ranelate l Promote osteoblast differentiation (#C7876-5)
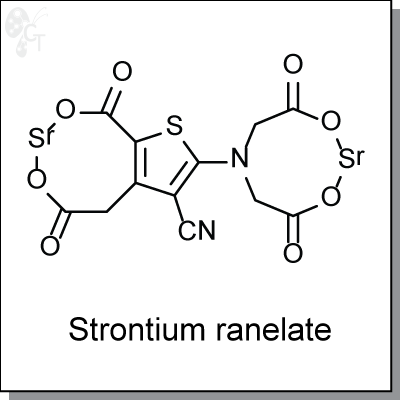
Strontium ranelate (Protelos), a strontium (II) salt of ranelic acid, is an agent for the treatment of osteoarthritis
and osteoporosis. Classified as a dual-action bone agent (DABA), strontium ranelate is unique in that it can
simultaneously inhibit bone resorption and stimulate bone formation. [1]
More specifically, strontium ranelate increases in vitro osteoblast differentiation from progenitors, as well as
osteoblast-induced osteoclastogenesis both in vitro and in vivo. Regarding bone-antiresorbing mechanisms,
strontium ranelate decreases osteoclast differentiation and activity, while increasing their apoptosis. [2]
|
Details
|
Chemical Formula:
|
|
C12H6N2O8SSr2
|
|
CAS No.:
|
|
135459-87-9
|
|
Molecular weight:
|
|
513.49
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
5-[Bis(carboxymethyl)amino]-2-carboxy-4-cyano-3-thiopheneacetic Acid
Strontium Salt
|
|
Solubility:
|
|
Up to 22mM in DMSO
|
|
Synonyms:
|
|
Strontium ranelate, Protelos, Osseor, Ranelic acid distrontium salt
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Ireland, G., Protos: The first dual-action bone agent for osteoporosis, JEMDSA, 2006, 11(1), 35.
2. Fonseca et al., Mechanism of action of strontium ranelate: what are the facts? Clin. Cases.
Mineral Bone Metabol. 2010, 7(1), 17-18. Pubmed ID: 22461285
|
Product Name: Ursolic (#C8776-10)
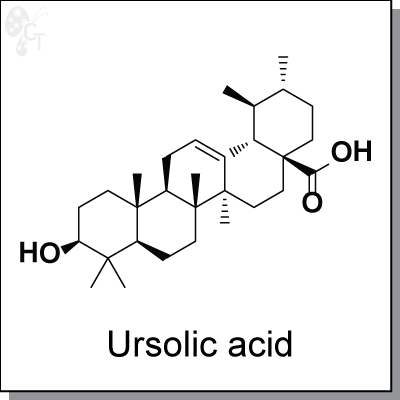
Ursolic acid is a natural compound enriched in apples. It is capable of inhibiting various types of cancer cells
by inhibiting the STAT3 activation pathway and human fibrosarcoma cells by reducing the expression of
matrix metalloproteinase-9 by acting through the glucocorticoid receptor. Ursolic acid reduced muscle
atrophy and stimulated muscle hypertrophy in mice by enhancing skeletal muscle insulin/IGF-I signaling
and inhibiting atrophy-associated skeletal muscle mRNA expression.
|
Details
|
Chemical Formula:
|
|
C30H48O3
|
|
CAS No.:
|
|
77-52-1
|
|
Molecular weight:
|
|
456.70
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical name:
|
|
(1S,2R,4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-
heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-
4a-carboxylic acid
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Shishodia S, et al. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents
through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of
cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003; 63 (15): 4375–83
2. Pathak AK, Bhutani M, Nair AS, et al. Ursolic acid inhibits STAT3 activation pathway leading to
suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol. Cancer Res.
2007; 5 (9): 943–55
3. Steven D. Kunkel, et al. mRNA Expression Signatures of Human Skeletal Muscle Atrophy Identify a
Natural Compound that Increases Muscle Mass. Cell Metabolism, 2011; Volume 13, Issue 6, 627-638.
|
|


