Disease Area, Lung Disease I
Product Name: Ambrisentan | Endothelin receptor type A/ETa antagonist (#C2627-5)
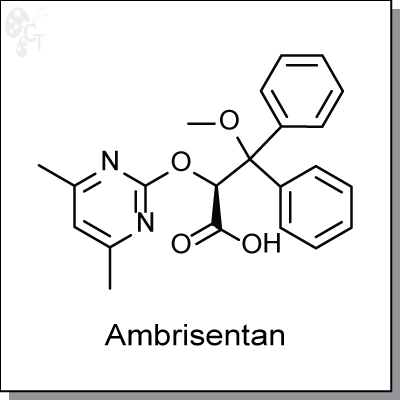
Ambrisentan is a propanoic acidbased, orally-available, endothelin receptor type A-receptor antagonist for
the once-daily treatment of pulmonary arterial hypertension (PAH). It has >4000-fold selectivity for the type A
receptor over type B receptor. Studies indicate that Ambrisentan has a low potential for drug interactions and
at therapeutic concentrations does not inhibit UDP-glucuronosyltransferases or CYPs, or induce MDR protein
2 or P-gp. (1)
In contrast with other similar therapies, Ambrisentan has been shown to have a lower potential for increase
of serum-level concentrations of aminotransferases. (2)
|
Details
|
Chemical Formula:
|
|
C22H22N2O4
|
|
CAS No.:
|
|
177036-94-1
|
|
Molecular weight:
|
|
378.42
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
white
|
|
Chemical name:
|
|
(S)-2-((4,6-dimethylpyrimidin-2-yl)oxy)-3-methoxy-3,3-diphenylpropanoic acid
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
LU-208075, BSF-208075
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC
|
1. Frampton, JE., Ambrisentan. Am. J. Cardiovasc. Drugs 2011, 11(4), 215-226. Pubmed ID: 21623643
2. Galie et al., Results of the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind,
Placebo-Controlled, Multicenter, Efficacy (ARIES) Study 1 and 2. Circulation, 2008, 117, 3010-3019. Pubmed
ID: 18506008
|
Product Name: BIBF1120 (Vargatef, Nintedanib) | VEGFR/PDGFR/FGFR inhibitor (#C2311-5)
.png)
BIBF1120 (Vargatef, Nintedanib) is an oxindole-based, orally-available triple kinase inhibitor targeting the
angiogenesis factors VEGF, PDGF, and FGF. It inhibits VEGFR, PDGFR, and FGFR at potency ranges of 13-
34, 59-65, and 37-108 nM. [1] Kinase panel studies show that it also inhibits Flt-3, Lck, Lyn, and Src at 26,
16, 195, and 156 nM, respectively. BIBF1120 also inhibits cell proliferation in MAPK and Akt signaling
pathways at EC50s of 10-80 nM.
Continuous oral QD dosing of BIBF1120 displayed antitumor activity in a number of tumor cell lines (FaDu,
Caki-1, HT-29, SKOV-3, Calu-6, PAC-120, and GS-9L.
BIBF-1120 is being explored as a treatment for idiopathic pulmonary fibrosis and has shown promise in the
delay of lung fibrosis progression. [2]
|
Details
|
Chemical Formula:
|
|
C31H33N5O4
|
|
CAS No.:
|
|
928326-83-4
|
|
Molecular weight:
|
|
539.62
|
|
Purity:
|
|
> 99%
|
|
Appearance:
|
|
Yellow
|
|
Chemical name:
|
|
(Z)-methyl 3-((4-(N-methyl-2-(4-methylpiperazin-1-yl)acetamido)phenylamino)
(phenyl)methylene)-2-oxoindoline-6-carboxylate
|
|
Solubility:
|
|
Up to 10 mM in DMSO
|
|
Synonyms:
|
|
BIBF-1120, BIBF 1120, BIBF1120, Vargatef, Nintedanib
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC
|
References
1. Lobera M, et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group.
(2013) Nat Chem Biol. 9(5):319-25. Pubmed ID: 23524983
|
Product Name: ICG-001 | Wnt signaling inhibitor (#C4001-5)
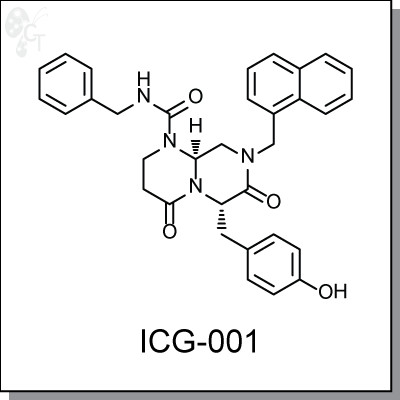
ICG-001 is a small-molecule antagonist of b-catenin/TCF-mediated transcription (IC50 = 3 uM) and
specifically downregulates the expression of a subset of b-catenin/TCF-responsive genes. ICG-001 binds
specifically to cyclic AMP response element-binding protein (CBP), therby disrupting interaction of CBP with
b-catenin. [1] ICG-001 selectively induces apoptosis in transformed, but not normal colon cells, and reduces
in vitro growth of colon carcinoma cells.
ICG-001 has also been shown to attenuate bleomycin-induced lung fibrosis in mice by selective inhibition of
Wnt/b-catenin-dependent transcription. [2]
ICG-001 is believed to correct defects in neuronal stem cell differentiation by inhibition of this pathway [3]
and associated polytopic protein Presenilin-1 (PS-1), which suggests potential therapies in Alzheimer's
disease.
The wnt/b-catenin signalling pathway has been implicated in regulation of cancer stem cells and ICG-001
has been studied in this regard. [4]
|
Details
|
Chemical Formula:
|
|
C33H32N4O4
|
|
CAS No.:
|
|
847591-62-2;780757-88-2
|
|
Molecular weight:
|
|
302.37
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Brown
|
|
Chemical name:
|
|
(R,2E,4E)-6-(4-(dimethylamino)benzoyl)-N-hydroxy-4-methylhepta-2,4-dienamide
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Synonyms:
|
|
Trichostatin A, TSA
|
|
Storage:
|
|
For longer shelf life, store solid powder or DMSO solution at -20oC desiccated.
|
References
1. Emami et al., A small molecule inhibitor of beta-catenin/CREB-binding protein transcription. Proc. Natl.
Acad. Sci. 2004, 101(34), 12682-12687. Pubmed ID: 15314234
2. Henderson et al., Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary
fibrosis. Proc. Natl. Acad. Sci. 2010, 107(32), 14309-14314. Pubmed ID: 20660310
3. Teo et al., Specific inhibition of CBP/beta-catenin interaction rescues defects in neuronal differentiation
caused by a presenilin-1 mutation. Proc. Natl. Acad. Sci. 2005, 102(34), 12171-12176. Pubmed ID: 16093313
4. Takahashi-Yanaga et al., Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin.
Cancer Res. 2010, 16, 3153-3162. Pubmed ID: 20660310
|
|

.png)
