Disease Area, Infectious Disease III
Product Name: Ormetoprim l Dihydrofolate (DHF) reductase (#C6763-10)
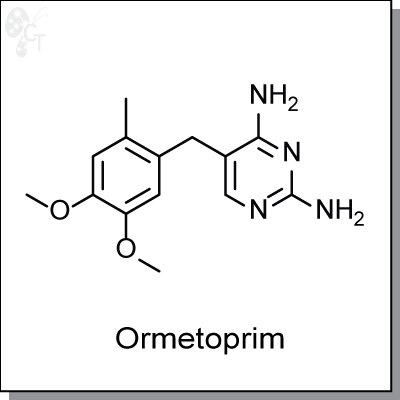
Ormetoprim is a diaminopyrimidine-based inhibitor of dihydrofolate reductase, the enzyme responsible for
NADPH-dependent reduction of 7,8-dihydrofolate to 5,6,7,8-tetrahydrofolate. (1) This inhibition results in the
interference in folic acid production. In bacteria, Ormetoprim, weakly antibacterial by itself, can act as a
potentiator by cotreatment with sulfadimethoxine, which in turn prevents the formation of folinic acid. (2)
|
Details
|
Chemical Formula:
|
|
C14H18N4O2
|
|
CAS No.:
|
|
6981-18-6
|
|
Molecular weight:
|
|
274.32
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
2,4-Diamino-5-(6-methylveratryl)pyrimidine
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
1. Manchand et al., Syntheses of Antibacterial 2,4-Diamino-5 benzylpyrimidines. Ormetoprim and
rimethoprim. J. Org. Chem. 1992, 57, 3531-3535.
2. Boothe, D.M., Sulfonamides and Sulfonamide Combinations, Merck Manuals, March, 2012.
|
Product Name: Plk-93 l Pl4K inhibitor (#C7930-5)
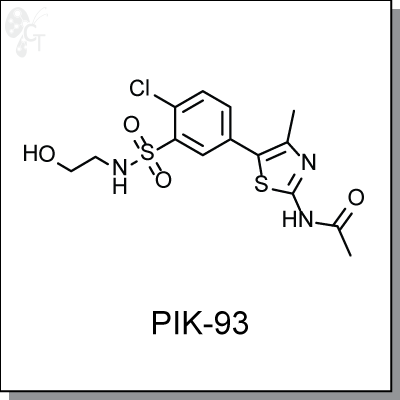
PIK93 is a phenylthiazole-based inhibitor of PI4KIIIb (IC50 = 19 nM) used to evaluate the role of PI4-K
isoforms in calcium signaling. [1] At a concentration of 300 nM PIK93 inhibited the p110g isoform of PI3K
(Class I) was inhibited at IC50 of 16 nM, while weakly inhibiting PI3K-C2a (IC50 = 16 uM). [2] PIK93 is
selective for PI4KIIIb over PI4KIIIa (EC90 = 250 nM and EC50 >10 uM, respectively) and is inactive toward
type II PI4Ks. [3] PIK93 was also identified as a potent anti-poliovirus and anti-HCV agent, with EC50s of
0.14 and 1.9 uM, respectively. [4]
|
Details
|
Chemical Formula:
|
|
C14H16ClN3O4S2
|
|
CAS No.:
|
|
593960-11-3
|
|
Molecular weight:
|
|
389.88
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
N-[5-[4-Chloro-3-[(2-hydroxyethyl)sulfamoyl]phenyl]-4-methylthiazol-2-yl]
acetamide
|
|
Solubility:
|
|
Up to 22 mM in DMSO
|
|
Synonyms:
|
|
PlK-93, PIK 93, PIK93
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Knight et al., A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling.
Cell, 2006, 125(4), 733-747. Pubmed ID: 16647110
2. Monet et al., Involvement of phosphoinositide 3-kinase and PTEN protein in mechanism of activation of
TRPC6 protein in vascular smooth muscle cells. J. Biol. Chem. 2012, 287(21), 17672-17681. Pubmed ID:
22493444
3. Toth et al., Phosphatidylinositol 4-kinase IIIbeta regulates the transport of ceramide between the
endoplasmic reticulum and Golgi. J. Biol. Chem. 2006, 281(47), 36369-36377. Pubmed ID: 17003043
4. Arita et al., Phosphatidylinositol 4-kinase III beta is a target of enviroxime-like compounds for
antipoliovirus activity. J. Virol. 2011, 85(5), 2364-2372. Pubmed ID: 22493444
|
Product Name: PSI-6206 (RO2433) | HCV RNA polymerase inhibitor (#C7620-2s)
.png)
PSI-6206 (RO2433) is the unphosphorylated parent compound of triphosphate analog PSI-7409, which is a
potent inhibitor of the HCV NS5B RNA dependent RNA polymerase. The monophosphate form of PSI-6206
was shown to be metabolized in primary human hepatocytes to its triphosphate analog PSI-7409.
Furthermore, the phosphoramidate prodrug of PSI-6206 monophosphate, PSI-7851, was developed.
Alternatively, PSI-6130, an aminated analog of PSI-6206 monophosphate, was also developed. (1,2,3)
PSI-7409, the triphosphate of PSI-6206 inhibits wild-type and S282T HCV RdRp with Ki values of 0.42 and
22 uM, respectively. PSI-7851, the phosphoramidate of PSI-6206 monophosphate, showed an EC50 value of
1.62 uM for inhibiting HCV RNA replication. (2)
|
Details
|
Chemical Formula:
|
|
C10H13FN2O5
|
|
CAS No.:
|
|
863329-66-2
|
|
Molecular weight:
|
|
260.22
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Clear Crystal
|
|
Chemical name:
|
|
(2'R)-2'-Deoxy-2'-fluoro-2'-methyluridine
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
PSI-6206, RO-2433, PSI6206, RO2433
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Rodriguez-Torres et al., Antiviral Activity, Pharmacokinetics, Safety, and Tolerability of PSI-7851, a Novel
Nucleotide Polymerase Inhibitor for HCV, Following Single and 3 Day Multiple Ascending Oral Doses in
Healthy Volunteers and Patients with Chronic HCV Infection. 60th AASLD Annual Meeting, 2009.
2. Murakami et al., The Mechanism of Action of b-D-2'-Deoxy-2'-Fluoro-2'-C-Methylcytidine Involves a
Second Metabolic Pathway Leading to b-D-2'-Deoxy-2'-Fluoro-2'-C-Methyluridine 5'-Triphosphate, a
Potent Inhibitor of the Hepatitis C Virus RNA-Dependent RNA Polymerase. Antimicrob. Agents. Chemother.
2008, 52(2), 458-464. Pubmed ID: 17999967
3. Ma et al., Characterization of the Metabolic Activation of Hepatitis C Virus Nucleoside Inhibitor b-D-2'-
Deoxy-2'-fluoro-2'-C-methylcytidine (PSI-6130) and Identification of a Novel Active 5'-Triphosphate
Species. J. Biol. Chem. 2007, 282, 29812-29820. Pubmed ID: 17698842
|
Product Name: RO318959 (Saquinavir) | HIV-1 protease inhibitor (#C7318-2s)
.png)
Saquinavir is a decahydroisoquinoline-based, selective inhibitor of aspartic protease encoded by HIV with an
IC50 range of 0.5-6.0 nM and IC90 range of 6.0-30.0 nM. [1] Binding inhibition constants for saquinavir
against HIV-1 and HIV-2 are 0.12 nM and
Antiviral potency of saquinavir were shown in JM cells infected with HIV-1 strain GB8 with a IC50 of 2.5 nM.
[2] Similarly, and IC50 of 2 nM was obtained against HIV-1 (strain RF) in C8166 cells.
Saquinavir was shown to be a substrate for P-glycoprotein transporter protein, affecting intracellular
concentrations and bioavailability. [3]
|
Details
|
Chemical Formula:
|
|
C38H50N6O5
|
|
CAS No.:
|
|
127779-20-8
|
|
Molecular weight:
|
|
670.84
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
(2S)-N-[(2S,3R)-4-[(3S)-3-(tert-butylcarbamoyl)-decahydroisoquinolin-2-yl]-3-
hydroxy-1-phenylbutan-2-yl]-2-(quinolin-2-ylformamido)butanediamide
|
|
Solubility:
|
|
Up to 22 mM in DMSO
|
|
Synonyms:
|
|
RO318959, RO-31-8959, saguinavir, Fortovase, Invirase
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Craig et al., Antiviral properties of Ro 31-8959, an inhibitor of human immunodeficiency virus (HIV)
proteinase. Anvitiral Res. 1991, 16, 295-395. Pubmed ID:1810306
2. Roberts et al., Rational design of peptide-based HIV proteinase inhibitors. Science, 1990, 248, 358-361.
Pubmed ID: 2183354
3. Kim et al., Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J. Pharmacol. Exp.
Ther. 1998, 286(3), 1439-1445. Pubmed ID: 9732409
|
|


.png)
.png)