Disease Area, Infectious Disease II
Product Name: Cefdinir l Antibiotic (#C2333-10)
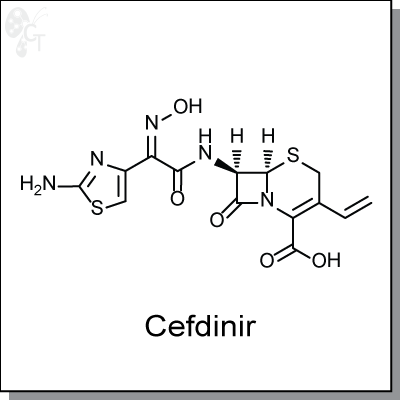
Cefdinir is an orally-available, b-lactam antibiotic that binds to penicillin-susceptible Staphylococcus aureus
2132 at an MIC of 25 nM (1) and E. Coli C11 at an MIC of 63 nM (ED50 of 1.32 mg/kg) (2) In contrast to other
oral cephalosporins, cefdinir retains substantial activity against a panel of bacterial strains and is found to be
extremely stable against numerous b-lactamases. Additionally, cefdinir inhibits the adherence of type-1
fimbriated E. coli to uroepithelial cells. (3)
In clinical studies, cefdinir has been shown to be superior to pencillin V and comparable to cephalexin and
amoxicillin/clavulanate in the treatment of both pediatric and adult bacterial infections. (3)
|
Details
|
Chemical Formula:
|
|
C14H13N5O5S2
|
|
CAS No.:
|
|
91832-40-5
|
|
Molecular weight:
|
|
395.41
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
(6R,7R)-7-[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(N-hydroxyimino)acetamido]-3-
ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
Cefdinir, FK482, Cl-983, and PD134393, Omnicef
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
1. Yamaguchi et al., In Vitro and In Vivo Antibacterial Activities of CS-834, a New Oral Carbapenem.
Antimicrob. Agents Chemother. 1998, 42(3), 555-563 Pubmed ID: 9517932
2. Fukuoka et al., Efficacy of CS-834 against Experimental Pneumonia Caused by Penicillin-Susceptible
and -Resistant Streptococcus pneumoniae in Mice. Antimicrob Agents Chemother. 1998, 42(1), 23-27.
Pubmed ID: 9449255
3. Guay et al., Cefdinir: An Expanded-Spectrum Oral Cephalosporin. Annals Pharmacother. 2000, 34,
1469-1477. Pubmed ID: 11144705
|
Product Name: Doripenem l Broad spectrum antibiotic (#C3674-10)
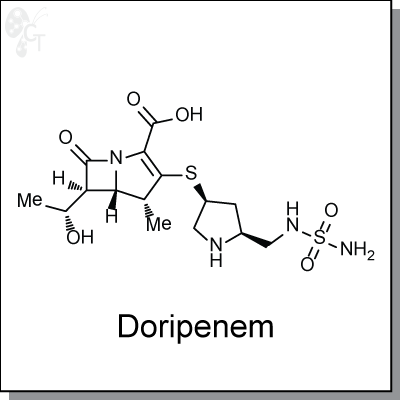
Doripenem is a parenterally-administred antimicrobial of the carbapenem family, with minimum inhibitory
concentrations (MIC) of 0.25 ug/mL in Pseudomonas aeruginosa and Escherichia coli. [1] Similar to other
b-lactam antibiotics, Doripenem forms stable acyl-enzymes with pencillin-binding proteins, leading to the
their inactivation and eventual cell wall rupture.
Doripenem is approved for the treatment of complicated intra-abdominal infections and urinary tract
infections. It is active against gram-positive, gram-negative, and anaerobic organisms, and is stable against
a wide variety of b-lactamases and renal dehydropeptidases. [2, 3]
|
Details
|
Chemical Formula:
|
|
C15H24N4O6S2.H2O
|
|
CAS No.:
|
|
148016-81-3
|
|
Molecular weight:
|
|
438.52
|
|
Purity:
|
|
> 99%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, 3-[[(3S,5S)-5-[[(aminosulfonyl)
amino]methyl]-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-,
hydrate
|
|
Solubility:
|
|
Up to 75 mM in DMSO
|
|
Synonyms:
|
|
Doripenem, Doribax
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Paterson et al., Doripenem. Clin. Infect. Dis. 2009, 49(2), 291-298. Pubmed ID: 19527173
2. Matthews et al., Doripenem monohydrate, a broad-spectrum carbapenem antibiotic.
Clin. Therapeutics, 2009, 31(1), 42-63. Pubmed ID: 19243706
3. Greer et al., Doripenem (Doribax): the newest addition to the carbapenems. Proceedings,
Baylor University Medical Center, 2008, 21(3), 337-341. Pubmed ID: 18628935
|
Product Name: GSK1349572 (Dolutegravir) | HIV integrase inhibitor (#C4134-2s)
.png)
GSK1349572, a novel tricyclic-core containing inhibitor, has an IC50 of 2.7 nM against HIV-1 with a
corresponding EC50s of 0.51 nM, 0.71 nM, and 2.2 nM in PBMCs, MT-4 cells, and PHIV assays,
respectively. [1] Cytotoxic concentration (CC50) in unstimulated and stimulated PBMCs was 189 uM and 52
uM, which results in a selectivity index of 9400. Though similar in potency to first-generation integrase
inhibitors, Raltegravir and Elvitegravir, GSK1349572 possesses and resistance profile that is markedly
different. In cross-resistance profiling experiments, GSK1349572 showed efficacy against five non-
nucleoside- and nucleoside- reverse transcriptase inhibitor-resistant viruses equal to that of wild-type virus
(EC50 = 1.3 to 2.1 nM). Similarly, GSK1349572 was efficacious against two protease inhibitor-resistant
viruses, again equivalent to activity against wild-type virus (EC50 = 0.36 and 0.37 nM).
In studies of clinical isolates from HIV-2-infected patients, the median EC50 value for GSK1349572 was found
to be 0.8 nM. [2]
|
Details
|
Chemical Formula:
|
|
C20H19F2N3O5
|
|
CAS No.:
|
|
1051375-16-6
|
|
Molecular weight:
|
|
419.38
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
4-[2-[(2R,3R)-3-(2,4-difluorophenyl)-3-hydroxy-4-(1,2,4-triazol-1-yl)butan-2-yl]
-1,3-thiazol-4-yl]benzonitrile
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
GSK1349572, S/GSK1349572, Dolutegravir
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Kobayashi et al., In Vitro Antiretroviral Properties of S/GSK1349572, a Next-Generation HIV Integrase
Inhibitor. Antimicrob. Agents. Chemother. 2011, 55(2), 813-821. Pubmed ID: 21115794
2. Charpentier et al., In vitro phenotypic susceptibility of HIV-2 clinical isolates to the integrase inhibitor
S/GSK1349572. AIDS, 2010, 24, 2753-2755. Pubmed ID: 20827161
|
Product Name: MK-0518 (Raltegravir) | HIV-1 integrase inhibitor (#C6051-5)
.png)
Raltegravir (Isentress)is an first-in-class, orally-available, oxadiazole-based inhibitor of HIV-1 integrase,
inhibiting strand transfer in vitro with an IC50 of 2-7 nM. [1] It is >1000-fold more selective for HIV-1 integrase
when compared to other phosphoryltransferases, such as the polymerase and RNase H activities of HIV-1
reverse transcriptase and the human polymerases alpha, beta, and gamma.
Raltegravir has a IC95 in human T lymphoid cell cultures of 31 nM and was also active against HIV-2 when
tested in CEMx174 cells, with an IC95 of 6nM. [2]
Raltegravir has been shown to be a potent and selective against Xenotropic murine leukemia-related
retrovirus (XMRV) at submicromolar concentrations in MCF-7 breast cancer (EC50 = 5 nM, EC90 = 3.5 uM)
and LNCaP prostate cancer (EC50 = 30 nM, EC90 0.46 um) cell lines. [3]
|
Details
|
Chemical Formula:
|
|
C20H21FN5O5
|
|
CAS No.:
|
|
871038-72-1
|
|
Molecular weight:
|
|
444.42
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
N-(4-fluorobenzyl)-5-hydroxy-1-methyl-2-(2-(2-methyl-1,3,4-oxadiazole-5-
carboxamido)propan-2-yl)-6-oxo-1,6-dihydropyrimidine-4-carboxamide
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
Raltegravir, MK-0518, MK0518, Isentress
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Temesgen et al., Raltegravir: first in class HIV integrase inhibitor. Ther. Clin. Risk Management, 2008,
4(2), 493-500. Pubmed ID: 18728839
2. Hicks et al., Raltegravir: the first HIV type 1 integrase inhibitor. Clin. Infect. Dis. 2009, 48(7), 931-939.
Pubmed ID: 19231980
3. Singh et al., Raltegravir is a potent inhibitor of XMRV, a virus implicated in prostate cancer and chronic
fatigue syndrome. PloS ONE 2010, 4(4), e9948. Pubmed ID: 20376347
|
|

.png)
.png)
