Far-red fluorescent protein Katushka2S
본문
|
Far-red fluorescent protein Katushka2S
- Super bright far-red fluorescence - high signal-to-noise ratio, fast maturation - Recommended for whole body imaging
Main properties 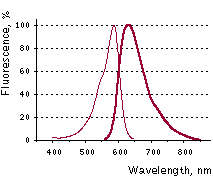 Katushka2S normalized excitation (thin line) and emission (thick line) spectra.
* Brightness is a product of extinction coefficient and quantum yield, divided by 1000.
Recommended filter sets and antibodies Katushka2S can be recognized using Anti-tRFP antibody (Cat.# AB233-AB234) available from Evrogen. Recommended Omega Optical filter sets are QMAX-Red and XF102-2. Katushka2S can also be detected using Texas Red filter sets or similar. For IVIS Lumina II imaging system the highest fluorescence signal for Katushka2S is observed with the following settings: In cell culture: "Cy5.5" (695-770 nm) channel using 570/35nm excitation In whole body imaging: "Cy5.5" (695-770 nm) channel using 605/35nm excitation
Performance and use Mammalian cells transiently transfected with Katushka2S expression vectors produce bright fluorescence in 12 hrs after transfection. No cytotoxic effects or visible protein aggregation are observed. Superior performance of Katushka2S in whole-body imaging was demonstrated using mouse xenograft model. HEK293FT cells transiently transfected with plasmids encoding different far-red fluorescent proteins were implanted into mice intramuscularly or subcutaneously. The cells were co-transfected with firefly luciferase to normalize the transfection efficiency and total numbers of injected cells. Katushka2S produced higher fluorescence signal and better signal-to-noise ratio than other far-red fluorescence proteins analyzed at various excitation and emission channels.
 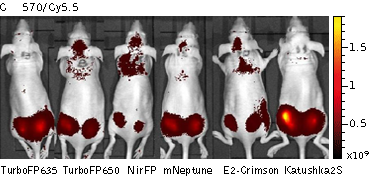 
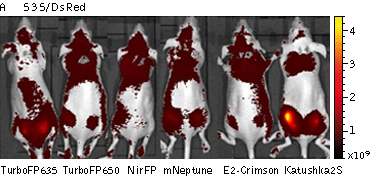
Whole-mouse imaging with IVIS Lumina II system (PerkinElmer). Representative fluorescence images of nude mice injected into the gluteal muscle with HEK293FT cells transiently expressing mNeptune, E2-Crimson, Katushka2S, TurboFP635, TurboFP650 and NirFP captured with indicated excitation (35-nm bandwidth centered at the indicated wavelength) and emission (DsRed 575- 650nm and Cy5.5 695-770nm) filter combination. Pseudocolor scale bar: radiant efficiency (photons/s)/ (µW/cm2). Note that scale bar differ for images with different excitation and emission filters. Images from [Luker et al., 2015]
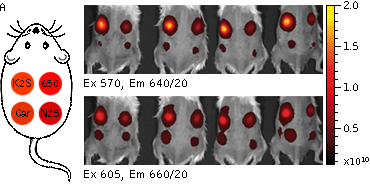
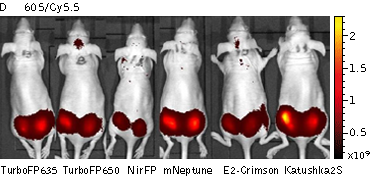
Whole-mouse imaging with IVIS Lumina II system (PerkinElmer). (A) Representative fluorescence images of mice injected in backs with HEK293FT cells transiently expressing Katushka2S (K2S), TurboFP650 (650), mCardinal (Car) and mNeptune2.5(N2.5). captured with indicated excitation (35-nm bandwidth centered at the indicated wavelength) and emission (640/20 and 660/20) filter combination. Pseudocolor scale bar: radiant efficiency (photons/s/cm2/sr)/(µW/cm2). (B) Graphs display mean values ±SEM for fluorescence radiant efficiency normalized to luciferase photon flux for each implant (n=4 per condition) for 570-nm (left) and 605-nm (right) excitation and listed emission filters. Fluorescent proteins are depicted by the following colors: Katushka2S (brown), TurboFP650 (pink), mCardinal (blue) and mNeptune2.5 (green). Images from [Luker et al., 2015]
Katushka2S was also compared with one of the best phytochrome photoreceptors iRFP720. Katushka2S produced brighter fluorescence intensity at its optimum conditions as compared with the best output from iRFP720. At the same time, both proteins showed comparable signal-to-noise ratios at the optimum wavelengths for excitation and emission since autofluorescence from mouse tissue was lower in the channel optimal for iRFP720.
Katushka2S was also compared with one of the best phytochrome photoreceptors iRFP720. Katushka2S produced brighter fluorescence intensity at its optimum conditions as compared with the best output from iRFP720. At the same time, both proteins showed comparable signal-to-noise ratios at the optimum wavelengths for excitation and emission since autofluorescence from mouse tissue was lower in the channel optimal for iRFP720.
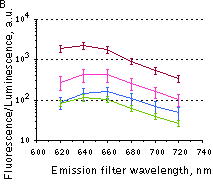
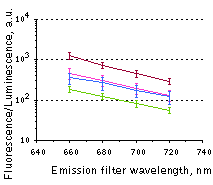
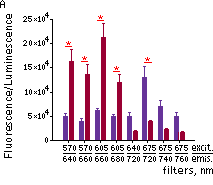
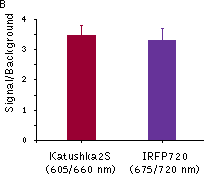
Fluorescence signals acquired with IVIS Spectrum system (PerkinElmer). Fluorescence signals normalized to corresponding firefly luciferase luminescence in mice injected in backs with HEK293FT cells transiently expressing Katushka2S or iRFP70. (A) Pairwise comparisons of the signals from Katushka2S (brown) and iRFP720 (violet) at different excitation/emission wavelengths. * p<0.0005 (B) Signal-to-noise ratios at optimal excitation and emission wavelengths for each protein.
pKatushka2S-C vector
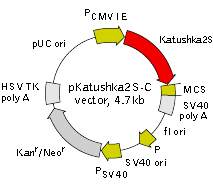
Vector description pKatushka2S-C is a mammalian expression vector encoding far-red fluorescent protein Katushka2S. The vector allows generation of fusions to the Katushka2S C-terminus and expression of Katushka2S fusions or Katushka2S alone in eukaryotic (mammalian) cells.
Katushka2S codon usage is optimized for high expression in mammalian cells (humanized) [Haas et al., 1996]. To increase mRNA translation efficiency, Kozak consensus translation initiation site is generated upstream of the Katushka2S coding sequence [Kozak, 1987]. Multiple cloning site (MCS) is located between Katushka2S coding sequence and SV40 polyadenylation signal (SV40 polyA).
The vector backbone contains immediate early promoter of cytomegalovirus (PCMV IE) for protein expression, SV40 origin for replication in mammalian cells expressing SV40 T-antigen, pUC origin of replication for propagation in E. coli, and f1 origin for single-stranded DNA production. SV40 polyadenylation signals (SV40 poly A) direct proper processing of the 3'-end of the reporter mRNA.
SV40 early promoter (PSV40) provides neomycin resistance gene (Neor) expression to select stably transfected eukaryotic cells using G418. Bacterial promoter (P) provides kanamycin resistance gene expression (Kanr) in E. coli. Kanr/Neorgene is linked with herpes simplex virus (HSV) thymidine kinase (TK) polyadenylation signals.
pKatushka2S-N vector
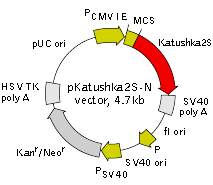
Vector description pKatushka2S-N is a mammalian expression vector encoding far-red fluorescent protein Katushka2S. The vector allows generation of fusions to the Katushka2S N-terminus and expression of Katushka2S fusions or Katushka2S alone in eukaryotic (mammalian) cells.
Katushka2S codon usage is optimized for high expression in mammalian cells (humanized) [Haas et al., 1996]. To increase mRNA translation efficiency, Kozak consensus translation initiation site is generated upstream of the Katushka2S coding sequence [Kozak, 1987]. Multiple cloning site (MCS) is located between Katushka2S coding sequence and SV40 polyadenylation signal (SV40 polyA).
The vector backbone contains immediate early promoter of cytomegalovirus (PCMV IE) for protein expression, SV40 origin for replication in mammalian cells expressing SV40 T-antigen, pUC origin of replication for propagation in E. coli, and f1 origin for single-stranded DNA production. SV40 polyadenylation signals (SV40 poly A) direct proper processing of the 3'-end of the reporter mRNA.
SV40 early promoter (PSV40) provides neomycin resistance gene (Neor) expression to select stably transfected eukaryotic cells using G418. Bacterial promoter (P) provides kanamycin resistance gene expression (Kanr) in E. coli. Kanr/Neorgene is linked with herpes simplex virus (HSV) thymidine kinase (TK) polyadenylation signals.
* The vector sequence has been compiled using the information from sequence databases, published literature, and other sources, together with partial sequences obtained by Evrogen. This vector has not been completely sequenced.
Generation of Katushka2S fusion proteins A localization signal or a gene of interest can be cloned into MCS of the vector. It will be expressed as a fusion to the Katushka2S C,N-terminus when inserted in the same reading frame as Katushka2S and no in- frame stop codons are present. Katushka2S-tagged fusions retain fluorescent properties of the native protein allowing fusion localization in vivo. Unmodified vector will express Katushka2S when transfectedinto eukaryotic (mammalian) cells.
Note: The plasmid DNA was isolated from dam+-methylated E. coli. Therefore some restriction sites are blocked by methylation. If you wish to digest the vector using such sites you will need to transform the vector into a dam- host and make fresh DNA.
Expression in mammalian cells pKatushka2S-C,N vector can be transfected into mammalian cells by any known transfection method. CMV promoter provides strong, constitutive expression of Katushka2S or its fusions in eukaryotic cells. If required, stable transformants can be selected using G418 [Gorman, 1985].
Propagation in E. coli Suitable host strains for propagation in E. coli include DH5alpha, HB101, XL1-Blue, and other general purpose strains. Plasmid incompatibility group is pMB1/ColE1. The vector confers resistance to kanamycin (30 μg/ml) to E. coli hosts. Copy number in E. coli is about 500.
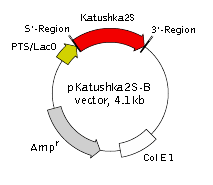
pKatushka2S-B vector
* The vector sequence has been compiled using the information from sequence databases, published literature, and other sources, together with partial sequences obtained by Evrogen. This vector has not been completely sequenced.
Vector description pKatushka2S-B is a prokaryotic expression vector encoding far-red fluorescent protein Katushka2S (see reporter description). Reporter codon usage is optimized for high expression in mammalian cells (humanized) [Haas et al., 1996].
The vector is primarily intended as a source of Katushka2S coding sequence. Flanking restriction sites are convenient for excision of Katushka2S sequence and its further insertion into other expression vectors of choice. Alternatively, Katushka2S coding sequence can be amplified by PCR.
Note: The plasmid DNA was isolated from dam+-methylated E. coli. Therefore some restriction sites are blocked by methylation. If you wish to digest the vector using such sites you will need to transform the vector into a dam- host and make fresh DNA.
The vector can be also used for Katushka2S expression in prokaryotes under the control of T5 promoter/lac operator. The vector backbone contains ColE1 origin of replication and ampicillin resistance gene for propagation and selection in E. coli.
Antibody against katushka2S(Anti-tRFP antibody) Rabbit polyclonal antibody against TurboRFP, TurboFP602, TurboFP635, Katushka2S, TurboFP650, NirFP, TagBFP, TagRFP, FusionRed, TagFP635, mKate2 and PA-TagRFP. DATA SHEET (PDF)
|
Ordering informations
|
Catalog No. |
Product Name |
Size |
|
pKatushka2S-C vector |
20 μg |
|
|
pKatushka2S-N vector |
20 μg |
|
|
pKatushka2S-B vector |
20 μg |
▣ 관련 페이지 ; Evrogen
댓글목록
등록된 댓글이 없습니다.