Cellular Mechanism, Cell Cycle II
Product Name: KU-55933 l ATM kinase inhibitor (#C5855-5)
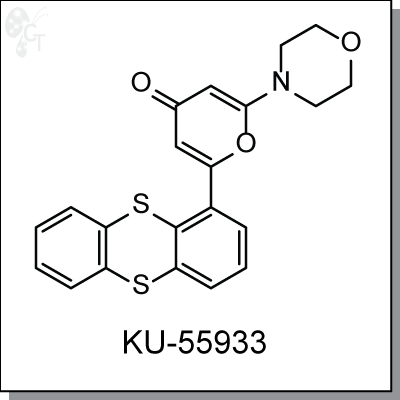
KU55933 is an ATP-competitive, thioanthrene-pyranone-based inhibitor of ATM kinase with an IC50 of 13 nM
and Ki of 2.2 nM and >100-fold selectivity over PI3K related kinases. [1] KU55933 inhibits cell proliferation by
inducing G1 cell cycle arrest by downregulation of cyclin D1 synthesis in MDA-MB-453 breast cancer cells
and PC-3 prostate cancer cells. [2] KU55933 inhibits insulin- and IGF1-stimulated Akt phosphorylation at both
Ser473 and Thr308. [2] ATM cellular inhibition by KU55933 was demonstrated in additional phosphorylation
targets, including p53 Ser15, H2AX Ser139, NBS1 Ser343, Chk1 Ser345, and SMC1 Ser966. [1]
KU55933 is being studied as a radiosensitizer, having been shown to sensitize HeLa cells to the effects of
topoisomerase inhibitors, etoposide, doxorubicin, amsacrine, and camptothecin. [1] When in combination
with rapamycin, KU55933 induces apoptosis and arrests any induced feedback activation of Akt. [2]
|
Details
|
Chemical Formula:
|
|
C21H17NO3S2
|
|
CAS No.:
|
|
587871-26-9
|
|
Molecular weight:
|
|
395.49
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
2-morpholino-6-(thianthren-1-yl)-4H-pyran-4-one
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
Ku-55933, KU 55933, KU55933
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
1. Hickson et al., Identification and characterization of a novel and specific inhibitor of the ataxia-
telangiectasia mutated kinase ATM. Cancer Res. 2004, 64(24), 9152-9159. Pubmed ID: 15604286
2. Li et al., The ATM inhibitor KU-55933 suppresses cell proliferation and induces apoptosis by blocking Akt
in cancer cells with overactivated Akt. Mol. Cancer. Ther. 2010, 9(1), 113-125. Pubmed ID: 20053781
|
Product Name: SP600125 l JNK2/JNk3 inhibitor (#C7760-5)
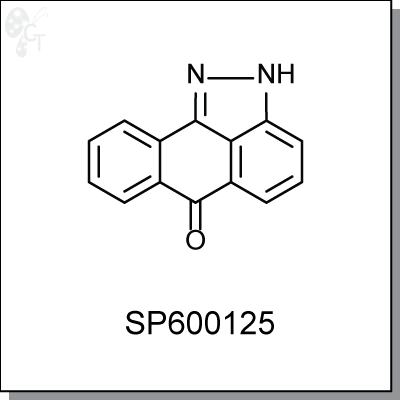
SP600125 is a reversible ATP-competitive, anthrapyrazolone-based inhibitor of JNK2 and JNK3, with IC50
values of 110 and 190 nM, respectively. In a selectivity panel, SP600125 was 10-fold selecxtive over MKK4,
25-fold selective over MKK3, MKK6, PKB, and PKCa, and 100-fold selective for all other kinases tested. [1]
SP600125 dose-dependently inhibits phosphorylation fo c-Jun and the expression of inflammatory genes
COX-2 (5 uM), IL-2 (6 uM), IFN-g (7 uM), and TNF-a (10 uM).
Independent of JNK activity, SP600125 has been shown to prevent the entry of cells into mitosis and leads to
endoreplication of DNA from the G2 phase. This inhibition predominantly occurs upstream of AIK and PLK1.
[2]
SP600125 has also been shown to be a ligand and antagonist of the aryl hydrocarbon receptor (AhR). In a
dose-dependent manner, SP600125 suppressed the inducdtion of CYP1A1 by TCDD and TCDD-induced AhR-
DNA complexes. Addition of SP600125 to cytosol just prior to TCDD addition completely suppresses AhR
transformation and DNA binding (IC50 ~ 7 uM) [3].
|
Details
|
Chemical Formula:
|
|
C14H8N2O
|
|
CAS No.:
|
|
129-56-6
|
|
Molecular weight:
|
|
220.23
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Yellow
|
|
Chemical name:
|
|
2H-Dibenzo[cd,g]indazol-6-one
|
|
Solubility:
|
|
Up to 100mM in DMSO
|
|
Synonyms:
|
|
SP600125, SP-600125, Pyrazolanthrone
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Bennett et al., SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci.,
2001, 98(24), 13681-13686. Pubmed ID: 11717429
2. Kim et al., SP600125 suppresses Cdk1 and induces endoreplication directly from G2 phase, independent of
JNK inhibition. Oncogene, 2010, 29(11), 1702-1716. Pubmed ID: 20062077
3. Joiakim et al., The Jun N-terminal kinase inhibitor SP600125 is a ligand and antagonist of the aryl
hydrocarbon receptor. Drug Metab. Dispos. 2003, 31(11), 1279-1282. Pubmed ID: 14570754
|
Product Name: VX-680 (Tozasertib) |Aurora A inhibitor (#C8916-10)
.png)
VX-680 (Tozasertib) is a small molecule Aurora kinase inhibitor (Aurora-A ~0.6nM, Aurora-B ~18nM, Aurora-
C ~4.6nM). VX-680 inhibits cell cycle progression, induces apoptosis and blocks tumor growth in varieties of
in vivo xenograft models, including leukemia, colon and pancreatic tumors.
|
Details
|
Chemical Formula:
|
|
C23H28N8OS
|
|
CAS No.:
|
|
639089-54-6
|
|
Molecular weight:
|
|
464.59
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Light yellow solid
|
|
Chemical name:
|
|
N-(4-((4-((5-methyl-1H-pyrazol-3-yl)amino)-6-(4-methylpiperazin-1-yl)pyrimidin-
2-yl)thio)phenyl)cyclopropanecarboxamide
|
|
Solubility:
|
|
Up to 100mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Elizabeth A Harrington, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases,
suppresses tumor growth in vivo. Nature Medicine 2004;10, 262 – 267.
2. Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005; 5(1):42-50.
|
|


.png)