Cellular Mechanism, Cell Cycle I
Product Name: Adriamycin (Doxorubicin) l DNA intercalator (#C2374-5)
.png)
Adriamycin (Doxorubicin) is an intravenous, anthracycline-based antibiotic and antineoplastic agent derived
from Streptomyces bacterium. Adriamycin enters cancer cell DNA and inhibits cell replication by arresting
protein synthesis.
In HK-2 cells, adriamycin decreases cell viability in a dose-dependent manner and induces an increase in
cells in the sub G1 and G2/M phases. It also increases secretion of TNFa, decreases expression of
phosphorylated PKA and Bcl-2, and increases phosphoryled signal transducer and activator of transcription
3, phospho-ERK,and ATF3. [1]
Due to adriamycin's route of administration and cytotoxic effects, extensive research has been conducted in
the area of assisted delivery of the chemotherapeutic (liposome [2], prodrug [3], polymer, gold-particles,
etc.)
|
Details
|
Chemical Formula:
|
|
C27H29NO11HCl
|
|
CAS No.:
|
|
25316-40-9
|
|
Molecular weight:
|
|
579.98
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Red Orange
|
|
Chemical name:
|
|
(8S,10S)-10-((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydro-2H-pyran-
2-yloxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-
tetrahydrotetracene-5,12-dione hydrochloride
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
Adriablastin, Adriblastin, Adriblastina, Adriamycin, Doxorubicine, Rubex,
Doxorubicinum, Doxorubicina, Doxil
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
1. Park et al., Doxorubicin induces cytotoxicity through upregulation of pERK-dependent ATF3. PLoSONE,
2012, 7(9), e44990. Pubmed ID: 23028726
2. Macmillan and Cancer Backup factsheet
3. Albright et al., Matrix metalloproteinase-activated doxorubicin prodrugs inhibit HT1080 xenograft growth
better than doxorubicin with less toxicity. Mol. Cancer Ther. 2005, 4, 751-760. Pubmed ID: 15897239
|
Product Name: AT7519 l CDK inhibitor (#C2875-5)
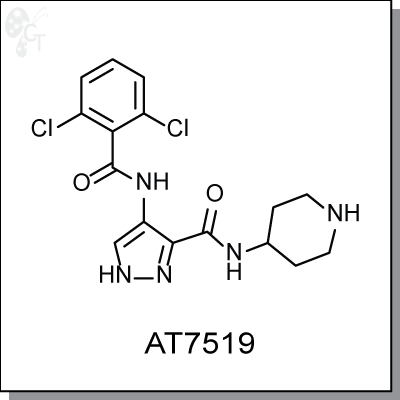
AT7519 is a pyrazole-based multi-cyclin dependent kinase inhibitor with IC50 activity against CDK2, CDK5,
and CDK9, of 47, 13, and In a panel of 26 human tumor cell lines, AT7519 showed potent antiproliferative
activity at 40-940 nM. 24h treatment of HCT116 cells showed phosphorylation inhibition of CDK1 substrate
PP1a (T320) and CDK2 substrates Rb (T821) and NPM (T199). (2)
|
Details
|
Chemical Formula:
|
|
C16H17Cl2N5O2
|
|
CAS No.:
|
|
902135-91-5
|
|
Molecular weight:
|
|
382.24
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Light Yellow
|
|
Chemical name:
|
|
4-(2,6-dichlorobenzamido)-N-(piperidin-4-yl)-1H-pyrazole-3-carboxamide
|
|
Solubility:
|
|
Up to 25 mM in DMSO
|
|
Synonyms:
|
|
AT7519, AT 7519, AT-7519
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Santo et al., AT7519, A novel small molecule multi-cyclin-dependent kinase inhibitor, induces apoptosis in
multiple myeloma via GSK-3b activation and RNA polymerase II inhibition. Oncogene, 2010, 29, 2325-2336.
Pubmed ID: 20101221
2. Squires et al., Biological characterization of AT7519, a small-molecule inhibitor of cyclin-dependent
kinases, in human tumor cell lines. 2009, 8, 324-332. Pubmed ID: 19174555
|
Product Name: AZD5438 |CDK 1/2/9 inhibitor (#C2543-5)
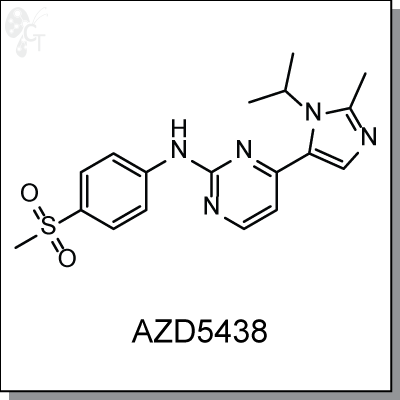
AZD5438 is an orally-bioavailable imidazole-pyrimidine inhibitor of cyclin dependent kinase (CDK) 1, 2, and
9 at IC50s of 16, 6, and 20 nM, respectively. In vitro AZD5438 showed significant antiproliferative activity in
human tumor cell lines with IC50s in the range of 0.2-1.7 uM, inhibiting phosphorylation of CDK substrates
pRb, nucleolin, protein phosphatase 1a, and RNA polymerase II carboxylate-terminus. AZD5438 blocks cell
cycling at the G2-M, S, and G1 phases and maintains suppression of biomarkers such as phospho-
pRbSer249/Thr252 for up to 16h following a single oral dose in SW620 cells. [1]
|
Details
|
Chemical Formula:
|
|
C18H21N5O2S
|
|
CAS No.:
|
|
602306-29-6
|
|
Molecular weight:
|
|
371.46
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Pale Orange
|
|
Chemical name:
|
|
4-(1-isopropyl-2-methyl-1H-imidazol-5-yl)-N-(4-(methylsulfonyl)phenyl)pyrimidin-
2-amine
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
AZD-5438, AZD 5438, AZD5438
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Byth et al., AZD5438, a potent oral inhibitor of cyclin-dependent kinases 1, 2, and 9, leads to
pharmacodynamic changes and potent antitumor effects in human tumor xenografts. Mol. Cancer. Ther.
2009, 8, 1856-1866. Pubmed ID: 19509270
|
Product Name: Docetaxel (taxotere) | Microtubules binder/stabilizer (#C3623-5)
.png)
Docetaxel, a semisynthetic analog of paclitaxel binds and stablizes tubulin, thus preventing microtubule
disassembly, resulting in cell-cycle arrest at the G2/M phase and inducing apoptosis. Docetaxel is known to
inhibit the anti-apoptotic gene Bcl2 and induces the expression of cell cycle inhibitor p27. (1)
In in vitro tubule formation assays, taxotere has an IC50 in the low nanomolar range. HUVEC migration
assays were more sensitive, with IC50s in the picomolar range in a chemokinetic assay. Furthermore,
HUVEC chemotaxis stimulated by either thymidine phosphorylase or vascular endothelial growth factor, was
inhibited by Taxotere in the double-digit picomolar range. (2)
Docetaxel has been studied extensively in combination with traditional anti-cancer agents (e.g. gemcitabine)
and more recently with newer generation agents such as cisplatin/5-fluorouracil (DCF), everolimus,
sunitinib, and antibody therapies. (1, 3)
|
Details
|
Chemical Formula:
|
|
C43H53NO14
|
|
CAS No.:
|
|
148408-66-6
|
|
Molecular weight:
|
|
807.88
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2R,3S)-3-((tert-
butoxycarbonyl)amino)-2-hydroxy-3-phenylpropanoyl)oxy)-4,6,11-trihydroxy-
4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-
methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
Docetaxel, Taxotere
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Nishiyama et al., Docetaxel: its role in current and future treatments for advanced gastric cancer. Gastric
Cancer 2009, 12, 132-141. Pubmed ID: 19890692
2. Hotchkiss et al., Inhibition of Endothelial Cell Function in Vitro and Angiogenesis in Vivo by Docetaxel
(Taxotere): Association with Impaired Repositioning of the Microtubule Organizing Center. Mol Cancer
Ther. 2002, 1, 1191-1200. Pubmed ID: 12479700
3. Fauzee et al., Taxanes: Promising Anti-Cancer Drugs. Asian Pacific J. Cancer Prev. 2011, 12, 837-851.
Pubmed ID: 21790213
|
|
.png)


.png)