Permanent Linkers, Photoactivatable Linkers
본문
|
Permanent Linkers, Photoactivatable Linkers
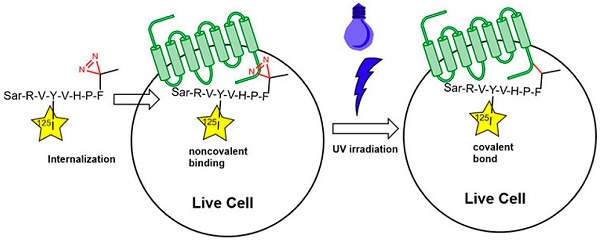
Irradiation of diazirines with UV light (ca. 350-360 nm) yields a highly reactive carbene species that can undergo insertions into C–C, C–H, O–H, and X–H (X = heteroatom) bonds of neighboring molecules to irreversibly form a covalent bond (Fig. 1). The diazirine moiety is the smallest of all photophores, so introduction of a diazirine-bearing amino acid into a peptide or protein usually does not impair its biological activity. Further advantages of diazirine crosslinkers are their stability at room temperature and their relative stability against nucleophiles as well as towards both acidic and basic conditions. Fig. 1: Use of photo-phenylalanine for the identification of angiotensin-II receptor binding sites; 125I is used as a radiotracer.
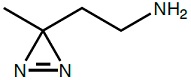
Photo-Ethylamine*HCl
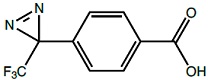
Photo-Benzoic acid
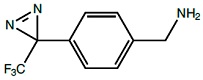
Photo-Benzylamine*HCl
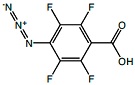
ATFB
|
Ordering informations
|
Catalog No. |
Product Name |
Size |
|
RL-2910 |
Photo-Ethylamine*HCl |
250, 500mg & 1g |
|
RL-2920 |
Photo-Benzoic acid |
200g & 1g |
|
RL-2930 |
Photo-Benzylamine*HCl |
200g & 1g |
|
RL-2035 |
ATFB |
250, 500mg, 1 & 5g |
▣ 관련 페이지 ; Iris Biotech GMBH
- 이전글Valine-Alanine-Based Enzymatically Cleavable Linkers 22.04.21
- 다음글Permanent Linkers with Maleimide Function 22.04.21
댓글목록
등록된 댓글이 없습니다.