Permanent Linkers with Maleimide Function
본문
|
Permanent Linkers with Maleimide Function
Michael addition of a thiol to a maleimide is commonly used for numerous bioconjugations. Several commercial constructs like Brentuximab vedotin, Trastuzumab emtansine, and Cimzia contain a thiol- maleimide adduct. However, this reaction is reversible. During the journey of an appropriate thioether containing drug through physiological media, this bond can break, and fragments are released which might contribute to certain unwanted or even toxic reactions.
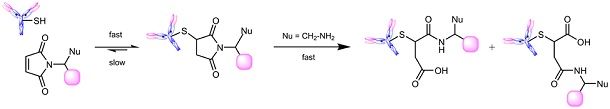
Fig. 1: Maleimides bind reversibly to thiols resulting in thioethers. This linkage turns stable once the maleimide ring is opened through hydrolysis.
However, if the succinimide moiety of a maleimide-thiol conjugate is hydrolyzed, the ring-opened product is fully stabilized towards cleavage (Fig. 1). The rates of ring-opening hydrolysis are greatly accelerated by electron withdrawing N-substituents and good nucleophiles in the proximity of the carbonyl functions. Thus, conjugates made with nucleophilic side chains and electron-withdrawing maleimides may be purposefully hydrolyzed to their ring-opened counterparts and ensure good in vivo stability.
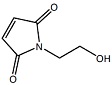
Mal-Et-OH
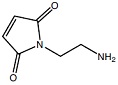
Mal-NH2*HCl
DBCO-mal
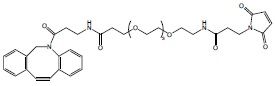
DBCO-PEG(4)-mal
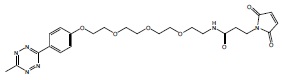
MeTz-PEG(4)-mal
|
Ordering informations
|
Catalog No. |
Product Name |
Size |
|
RL-3000 |
Mal-Et-OH |
1 & 5g |
|
RL-2780 |
Mal-NH2*HCl |
250mg, 1 & 5g |
|
RL-2490 |
DBCO-mal |
25, 100mg & 1g |
|
RL-2500 |
DBCO-PEG(4)-mal |
10, 25, 100 & 500mg |
|
RL-2340 |
MeTz-PEG(4)-mal |
10, 25 & 100mg |
▣ 관련 페이지 ; Iris Biotech GMBH
- 이전글Permanent Linkers, Photoactivatable Linkers 22.04.21
- 다음글Permanent Linkers, Hydrophobic Spacer Molecules 22.04.21
댓글목록
등록된 댓글이 없습니다.