Disease Area, Oncology I
Product Name: A-674563 l PKB/Akt inhibitor (#C2167)
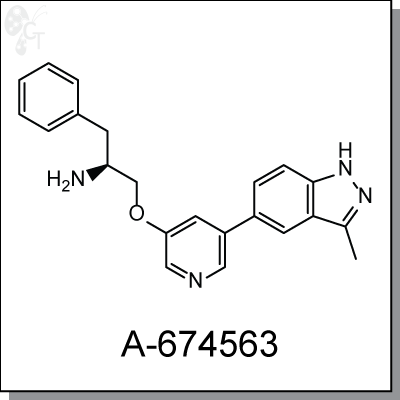
A-674563 is a potent and selective PKB/Akt inhibitor with an IC50 of 14 nM. It binds to ATP-binding site of Akt
and reduces the phosphorylation of Akt's downstream targets (GSK3α/β, FOXO3, TSC2, mTOR and S6, etc.).
A-674563 also inhibits activity of PKA and CDK2 with IC50 of 16 and 46 nM, respectively. A-674563 inhibits
proliferation of tumor cells with IC50 of 0.4 µM1.
In STS cells, A-674563 induces Akt pathway blockade that decreases downstream target phosphorylation,
and induces G2 cell cycle arrest and apoptosis. In vivo, A-674563 reduces STS xenograft growth2. When
given in combination, A-674563 enhanced the efficacy of paclitaxel in a PC-3 xenograft model1.
|
Details
|
Chemical Formula:
|
|
C22H22N4O
|
|
CAS No.:
|
|
552325-73-2
|
|
Molecular weight:
|
|
358.44
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical name:
|
|
(2S)-1-(5-(3-methyl-1H-indazol-5-yl)pyridin-3-yloxy)-3-phenylpropan-2-amine
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
1. Furuta et al., T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral
infections. Antiviral Res. 2009, 82, 95-102. Pubmed ID: 19428599
2. Kiso et al., T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc. Natl. Acad. Sci.
2010, 107(2), 882-887. Pubmed ID: 20080770
|
Product Name: Abiraterone l P450 CYP17 inhibitor (#C2247)
|
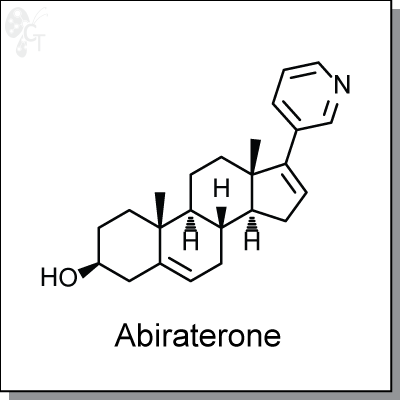
Abiraterone is a potent steroidal inhibitor against cytochrome P450 17alpha-hydroxylase-17,20-lyase (CYP17)
with an IC50 at 4 nM. It was approved by the FDA in April 2011 to treat castration-resistant prostate cancer. A
phase I/II clinical trial evaluating abiraterone acetate in advanced breast cancer patients are also underway.
In preclinical studies, abiraterone has demonstrated the ability to selectively inhibit the target enzyme,
resulting in inhibition of testosterone production in both the adrenals and the testes.
|
Details
|
Chemical Formula:
|
|
C24H31NO
|
|
CAS No.:
|
|
154229-19-3
|
|
Molecular weight:
|
|
349.51
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical name:
|
|
(3S,8R,9S,10R,13S,14S)-10,13-dimethyl-17-(pyridin-3-yl)-
2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Attard G et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of
castration-resistant prostate cancer. J Clin Oncol. 2009;27(23):3742-8.
2. Attard G et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that
castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563-71.
|
Product Name: ABT-263 (Navitoclax) | Bcl-2 family inhibitor (#C2263)
.png)
ABT-263 is an orally-available Bcl-2 family inhibitor with Ki values of < 1 nM for Bcl-2, Bcl-xL, and Bcl-w.
ABT-263 disrupts key Bcl interactions with proteins such as Bim, inducing apoptosis. Despite being a Bad-
like Bh3 mimetic, ABT-263 has been shown to possess cytotoxic activity and induce apoptosis based
primarily on its Bcl-2 family inhibitory activity.
ABT-263 as a standalone agent has modest activity in lymphoma and myeloma xenografts, but is extremely
effective in enhancing the efficacy of clinically relevant therapies such as rituxumab and bortezomib. (1)
In a panel of small cell lung cancer (SCLC) xenografts, including H146, H889, and H1963 models, ABT-263
displays excellent antitumor effects, leading to tumor regression. (2)
|
Details
|
Chemical Formula:
|
|
C47H55ClF3N5O6S3
|
|
CAS No.:
|
|
923654-51-6
|
|
Molecular weight:
|
|
947.61
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
(R)-4-(4-((4'-chloro-4,4-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)
piperazin-1-yl)-N-((4-((4-morpholino-1-(phenylthio)butan-2-yl)amino)-3-
((trifluoromethyl)sulfonyl)phenyl)sulfonyl)benzamide
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
ABT-263, Navitoclax, ABT263, ABT 263
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Tse et al., ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008, 68,
3421-3428. Pubmed ID: 18451170
2. Shoemaker et al., Activity of the Bcl-2 Family Inhibitor ABT-263 in a Panel of Small Cell Lung Cancer
Xenograft Models. Clin. Cancer Res. 2008, 14, 3268-3277. Pubmed ID: 18519752
|
Product Name: ABT-737 | Apoptosis inhibitor (#C2281)
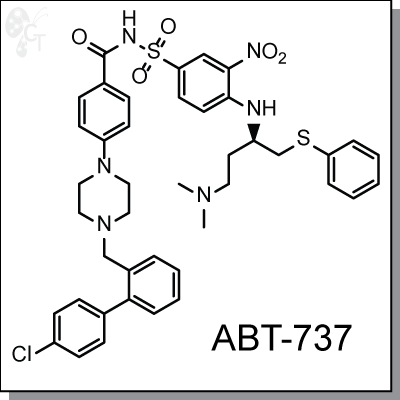
ABT-737 is a selective and potent small molecule inhibitor of protein Bcl-2, Bcl-XL, and Bcl-w. Like a BAD
BH3 peptide, ABT-737 binds to and antagonizes anti-apototic Bcl-2 family proteins instead of directly
activating the apoptotic process. ABT-737 binds with high affinity (ki < 1nm) to Bcl-XL, Bcl-2 and Bcl-w, but
not to Bcl-B, Mcl-1 and Al protein.
ABT-737 displays a wide range of single-agent activity against cells from lymphoma, leukemia, solid tumor
SCLC, and primary cells derived from patient. In animal models, ABT-737 causes tumor regression and
improves survival1-2. ABT-737 has shown synergistic anti-tumor activity when used together with vorinostat
or bemicitabine3,4.
|
Details
|
Chemical Formula:
|
|
C42H45ClN6O5S2
|
|
CAS No.:
|
|
852808-04-9
|
|
Molecular weight:
|
|
813.43
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Off White solid
|
|
Chemical name:
|
|
4-[4-[(4'-Chloro[1,1'-biphenyl]-2-yl)methyl]-1-piperazinyl]-N-[[4-[[(1R)-3-
(dimethylamino)-1-[(phenylthio)methyl]propyl]amino]-3-nitrophenyl]sulfonyl]
benzamide
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution
at -20oC
|
References
1. Oltersdorf, T. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature.
2005 Jun 2;435(7042):677-81.
2. Van Deelft , MF. et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces
apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006 Nov;10(5):389-99.
3. Zhang, C. et al. Synergistic anti-tumor activity of gemcitabine and ABT-737 in vitro and in vivo through
disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther. 2011 Jul;10(7):1264-75.
4. Wiegmans AP, et al. Deciphering the molecular events necessary for synergistic tumor cell apoptosis
mediated by the histone deacetylase inhibitor vorinostat and the BH3 mimetic ABT-737. Cancer Res. 2011
|
|


.png)
