|
Disease Area, Immunology IV
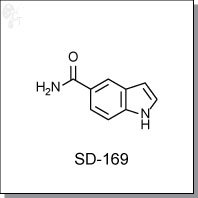
Product Name: SD-169 l p38 MAPK inhibitor
 |
|
SD169 is an indole-5-carboxamide, orally-available, ATP-competitive, a-selective p38 MAPK inhibitor that
targets a wide variety of inflammatory cells, including neutrophils, monocytes, macrophages, B and CD4+ T
cells, and endothelial cells. [1] Through interactions with Schwann cell and TNF activity, SD169 promotes
axonal regeneration in peripheral nerves. [2]
SD169 has been shown to reduce myeloma-induced osteolytic bone lesions and restored bone mass by
downregulating osteoclastogenesis and osteoblastogenesis in xenografted primary myeloma-SCID-hu or
myeloma cell line-SCID mouse models. [3] SD169 was also shown downregulates pp38 in myeloma cells in
vitro and in vivo.
In the diabetes therapeutic area, SD169 significantly reduces p38 and HSP60 expression in T cells of the
pancreatic beta islets. In studies using hyperglycemic NOD mice, SD169 treatment lowered blood glucose
and improved glucose homeostasis.
|
Details
|
Chemical Formula:
|
|
C9H8N2O
|
|
CAS No.:
|
|
1670-87-7
|
|
Molecular Weight:
|
|
160.17
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
1H-indole-5-carboxamide
|
|
Solubility:
|
|
Up to 22 mM in DMSO
|
|
Synonyms:
|
|
SD-169, Sd169
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Medicherla et al., p38 MAPK inhibition reduces diabetes-induced impairment of wound healing. Diabetes,
Metab. Syndr. Obes. 2009, 2, 91-100.
2. Myers et al., Inhibition of p38 MAP kinase activity enhances axonal regeneration. Exp. Neurol. 2003, 184,
606-614.
3. Yang et al., Constitutive activation of p38 MAPK in tumor cells contributes to osteolytic bone lesions in
multiple myeloma. Leukemia, 2012, 26, 2114-2123.
4. Medicherla et al., J. Pharmacol. Exp. Ther. 2006, 318(1), 99-107.
|
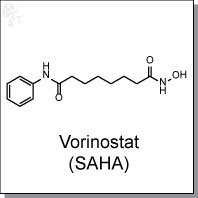
Product Name: Vorinostat (SAHA) l Class I&II HDAC inhibitor
 |
|
Vorinostat (SAHA) is a potent inhibitor of Classes I and II histone deacetylases (HDACs) that works by
chelating Zinc ions found in the active site of HDACs. Vorinostat's inhibition of HDAC activity results in the
accumulation of acetylated histones and acetylated proteins, including transcription factors crucial for the
expression of genes needed to induce cell differentiation.
Vorinostat inhibits the proliferation of both normal cells and a wide variety of transformed cells. It induces
death in tumor cells while leaves normal cells alive. Vorinostat inhibits tumor growth in a variety of animal
models1. Marketed under the name Zolinza, Vornostat is a FDA approved medicine for the treatment of
cutaneous T cell lymphoma (CTCL). Vorinostat is now under clinical investigations for several other types of
cancers and for its potential use in eradicating HIV from HIV+ patients2,3.
|
Details
|
Chemical Formula:
|
|
C14H20N2O3
|
|
CAS No.:
|
|
149647-78-9
|
|
Molecular Weight:
|
|
264.32
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical Name:
|
|
N-hydroxy-N'-phenyl-octanediamide
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Richon VM. Cancer biology: mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic
acid), a novel histone deacetylase inhibitor. Br J Cancer. 2006 Dec95(S1): S2–S6.
2. Contreras X, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol
Chem. 2009 284 (11): 6782–9.
3.Archin,NM. et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy.
Nature. 2012 Jul 25;487(7408):482-5.
|
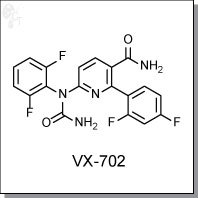
Product Name: VX-702 l P38 MAPK inhibitor
 |
|
VX-702 is an orally-available, aminopyridine-based, ATP-competitive inhibitor of p38 MAPK with a Kd of 3.7
nM and 17 nM at 10 uM for p38a and p38b, respectively. In an ex vivo blood assay primed with LPS< VX-702
dose-dependently inhibited the production of IL-6, IL-1b, TNFa at IC50 of 59, 122, and 99 ng/mL, respectively.
VX-702 was found to be equivalent to prednisolone and methotrexate in a mouse collagen-induced arthritis
model.
Clinical efficacy models plus transient suppression of inflammation biomarkers suggest that p38 MAPK
inhibition by agents such as VX-702 may not be a viable approach to the treatment of chronic inflammation in
RA.
|
Details
|
Chemical Formula:
|
|
C19H12F4N4O2
|
|
CAS No.:
|
|
479543-46-9
|
|
Molecular Weight:
|
|
404.32
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
1-(5-carbamoyl-6-(2,4-difluorophenyl)pyridin-2-yl)-1-(2,6-difluorophenyl)urea
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
VX-702. VX702
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Goldstein et al., Selective p38alpha inhibitors clinically evaluated for the treatment of chronic inflammatory
disorders. J. Med. Chem. 2010, 53, 2345-2353.
2. Ding, C., Drug evaluation: VX-702, a MAP kinase inhibitor for rheumatoid arthritis and acute coronary
syndrome. Curr. Opin. Invest. Drugs, 2006, 7(11), 1020-1025.
3. Damjanov et al., Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in
rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis
Rheum., 2009, 60(5), 1232-1241.
|
Ordering informations
|


