|
Disease Area, Immunology III
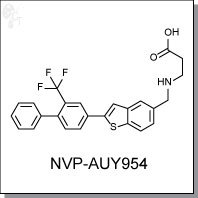
Product Name: NVP-AUY954 l S1P1 agonist
 |
|
NVP-AUY954 is an orally-available, benzothiazole-based, monoselective agonist of the sphingosine-1-
phosphate receptor 1 (S1P1) intended for the treatment of human autoimmune mediated inflammatory
neuropathies. Along with EC50 potency of 1.2 nM, NVP-AUY954 has at least 280-fold selectivity for S1P1
compared to the receptor's four other subtypes.
NVP-AUY954 induces a potent and reversible reduction of circulating lymphocytes; in combination with
Everolimus, NVP-AUY954 can prolong the survival of cardiac allografts in a rat transplantation model. NVP-
AUY954 also has been shown to activate downstream kinase cascades, increasing Erk (Tyr204) and Akt
(Ser473) phosphorylation at EC50s of 0.1 and 1.0 nM, respectively.
NVP-AUY954 efficacy has been correlated with an accumulation of plasmacytoid dendritic cells (pDC), which
may have therapeutic value in the treatment of multiple sclerosis.
|
Details
|
Chemical Formula:
|
|
C25H20F3NO2S
|
|
CAS No.:
|
|
N/A
|
|
Molecular Weight:
|
|
455.12
|
|
Purity:
|
|
> 99%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
3-[[2-[4-phenyl-3-(trifluoromethyl)phenyl]-1-benzothiophen-5-yl]methylamino]
propanoic acid
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Synonyms:
|
|
NVP-AUY954, AUY954, AUY-954
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Zhang et al., AUY954, a selective S1P1 modulator, prevents experimental autoimmune neuritis. J.
Neuroimmunol. 2009, 216, 59-65.
2. Pan et al., A Monoselective Sphingosine-1-Phosphate Receptor-1 Agonist Prevents Allograft Rejection in a
Stringent Rat Heart Transplantation Model. Chem. Biol. 2006, 13, 1227-1234.
3. Galicia-Rosas et al., A Sphingosine-1-Phosphate Receptor 1-Directed Agonist Reduces Central Nervous
System Inflammation in a Plasmacytoid Dendritic Cell-Dependent Manner. J. Immunol. 2012, 189, 3700-3706.
|
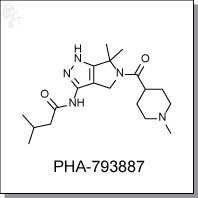
Product Name: PHA-793887 l CDK2/5/7 inhibitor
 |
|
PHA-793887 is an intravenously-administered, inhibitor of cyclin dependent kinases CDK2, CDK5, CDK7, at
IC50s of 8 nM, 5 nM, and 10 nM, respectively. [1] As a multi-CDK isoform inhibitor, it was postulated that
PHA-793887 might overcome resistance-based mechanisms and lead to enhanced tumor suppression. PHA-
793881 was found in Phase I dose-ranging studies to induce severe dose-related hepatotoxicity, thus halting
clinical studies.
Additionally, PHA-793887 was found to impair TLR signaling, thus suppressing cytokine production (type 1
interferon, interleukin-6, -10, -12, and TNFa), thereby increasing susceptibility of cancer patients to diseases
such as herpes viridae. [3]
PHA-793887 was found to be an effective tool compound for the monitoring of the E2F gene signature
pathway.
|
Details
|
Chemical Formula:
|
|
C19H31N5O2
|
|
CAS No.:
|
|
718630-59-2
|
|
Molecular Weight:
|
|
361.48
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Off White
|
|
Chemical Name:
|
|
N-(6,6-dimethyl-5-(1-methylpiperidine-4-carbonyl)-1,4,5,6-tetrahydropyrrolo[3,4-c]
pyrazol-3-yl)-3-methylbutanamide
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
PHA-793887, PHA 793887, PHA793887
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Brasca et al. Optimization of 6,6-dimethyl pyrrolo[3,4-c]pyrazoles: Identification of PHA-793887, a potent
CDK inhibitor suitable for intravenous dosing. Bioorg Med Chem, 2010, 18(5), 1844-1853.
2. Massard et al., A first in man, phase I dose-escalation study of PHA-793887, an inhibitor of multiple cyclin-
dependent kinases (CDK2, 1 and 4) reveals unexpected hepatotoxicity in patients with solid tumors. Cell
Cycle 2011, 10(6), 963-970.
3.Zoubir et al., An inhibitor of cyclin-dependent kinases suppresses TLR signaling and increases the
susceptibility of cancer patients to herpesviridae. Cell Cycle 2011, 10(1), 118-126.
4.Locatelli et al., Transcriptional analysis of an E2F gene signature as a biomarker of activity of the cyclin-
dependent kinase inhibitor PHA-793887 in tumor and skin biopsies from a phase I clinical study. Mol. Cancer Ther. 2010, 9(5), 1265-1273
|
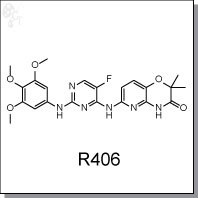
Product Name: R406 l SYK inhibitor
 |
|
R788 is an orally-available, ATP-competitive, methylene phosphate prodrug of R406, which is a potent
inhibitor of Syk (IC50 = 41 nM). [1] In a broad panel of receptor, ion channel, and enzyme binding assays,
R406 was shown to inhibit adenosine A3 receptor (IC50 = 81 nM), adenosine transporter (IC50 = 1.8 uM), and
monoamine transporter (IC50 = 2.7 uM). Followup measurements for ligand-induced guanosine 5'-O-
(thiotriphosphate) binding to the adenosine A3 receptor showed that R406 possesses antagonistic activity
with an IC50 of 93 nM. R406 inhibits phosphorylation of Syk substrate linker for activation of T cells in mast
cells and B-cell linker protein/SLP65 in B cells. R406 does not inhibit phosphorylation of Sky tyrosine 352, but
does inhibit phosphorylation of LAT tyrosine 191. R406 also inhibits IgE- and IgG-mediated activation of Fc
receptor signaling.
In a large panel of diffuse large B-cell lymphoma cell lines, R406 inhibited cellular proliferation at EC50s
ranging from 0.8 to 8.1 uM.
|
Details
|
Chemical Formula:
|
|
C23H24FN6O9P.2Na
|
|
CAS No.:
|
|
1025687-58-4
|
|
Molecular Weight:
|
|
624.42
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
2H-Pyrido[3,2-b]-1,4-oxazin-3(4H)-one, 6-[[5-fluoro-2-[(3,4,5-trimethoxyphenyl)
amino]-4-pyrimidinyl]amino]-2,2-dimethyl-4-[(phosphonooxy)methyl]-, sodium
salt (1:2)
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Braselmann S., et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling
and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006; 319(3):998-1008.
2. Sweeny DJ, Li W, Grossbard E, Lau DT. Contribution of gut bacteria to the metabolism of the spleen
tyrosine kinase (Syk) inhibitor R406 in cynomolgus monkey. Xenobiotica. 2010;40(6):415-23.
|
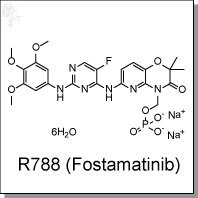
Product Name: R788 (Fostamatinib disodium) l Orally active Syk inhibitor
 |
|
Leukadherin-1 (LA1), a novel small molecule agonist of integrin CD11b/CD18, can increase the extent of
CD11b/CD18-dependent cell adhesion of transfected cells and of primary human and mouse neutrophils
(EC50 =4µM in vitro), which resulted in decreased chemotaxis and transendothelial migration. LA1 can also
decrease leukocyte recruitment and reduced arterial narrowing after injury in rats. Moreover, compared to a
known integrin antagonist, LA1 preserved kidney function better in a mouse model of experimental nephritis.
It inhibited leukocyte recruitment by increasing leukocyte adhesion to the inflamed endothelium, which was
reversed with a blocking antibody.
|
Details
|
Chemical Formula:
|
|
C22H15NO4S2
|
|
CAS No.:
|
|
344897-95-6
|
|
Molecular Weight:
|
|
421.49
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical Name:
|
|
(Z)-4-(5-((3-benzyl-4-oxo-2-thioxothiazolidin-5-ylidene)methyl)furan-2-yl)benzoic acid
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Synonyms:
|
|
Fostamatinib disodium, Fostamatinib, R788, R935788
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Braselmann et al., R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling
and reduces immune complex-mediated inflammation. J. Pharma. Exp. Ther. 2006, 319(3), 998-1008.
2. Chen et al., SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-
cell lymphoma. Blood 2008, 111, 2230-2237. Pubmed ID: 18006696
|
|



