|
Disease Area, Cardiovascular & Metabolic V
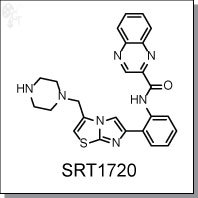
Product Name: SRT1720 | SIRT inhibitor
|
|
|
SRT1720 is a selective activator of human SIRT1 (0.16 µM and 781%) versus the closest sirtuin homologues,
SIRT2 and SIRT3. It binds to the SIRT1 enzyme-peptide substrate complex at an allosteric site amino-
terminal to the catalytic domain and lower the Michaelis constant for acetylated substrates. In animal studies
SRT1720 was found to improve insulin sensitivity and lower plasma glucose levels in fat, muscle and liver
tissue, and increased mitochondrial and metabolic function. The claim that SRT1720 is a SIRT1 activator has
been questioned and further defended since 2007.
|
Details
|
Chemical Formula:
|
|
C25H23N7OS
|
|
CAS No.:
|
|
1001645-58-4
|
|
Molecular Weight:
|
|
469.56
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical Name:
|
|
N-(2-(3-(piperazin-1-ylmethyl)imidazo[2,1-b]thiazol-6-yl)phenyl)quinoxaline-2-
carboxamide
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
References:
1. Milne JC et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes.
Nature. 2007;450(7170):712-6
2. Michelle Pacholec et al. SRT1720, SRT2183, SRT1460, and Resveratrol Are Not Direct Activators of SIRT1. J
Biol Chem. 2010; 285(30)
3. Beher D et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des 2009. 74
(6): 619–24
4. K. Zarse et al. Differential Effects of Resveratrol and SRT1720 on Lifespan of AdultCaenorhabditis elegans.
Hormone and Metabolic Research; 2009.42 (12): 837–839
5. Dai H et al. "SIRT1 activation by small molecules - kinetic and biophysical evidence for direct interaction of
enzyme and activator. J Biol Chem 2010;285 (43): 32695–32703
|
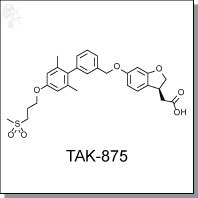
Product Name: TAK-875 | GPR40 agonist
|
|
|
TAK-875 is an orally-available, benzofuran-based, agonist of GPR40 for the potential treament of Type 2
diabetes mellitus, with an human EC50 of 0.014 uM in a FLIPR assay. It has high selectivity over GPR41,
GPR43, and GPR120, with EC50s all greater than 10 uM. Oral dosing (0.3-3 mg/kg) of TAK-875 in a
glucose intolerance test in female Wistar rats reduced blood glucose excursion. Insulin secretion was
increased during an oral glucose tolerance test.
TAK-875 was shown to activate the Gqa-mediated signalling pathway in pancreatic b-cells. Prolonged
agonist stimulation by TAK-875 revealed no evidence of b-cell dysfunction or toxicity, nor does it cause a
induction of a marker of apoptosis in pancreatic b-cells.
|
Details
|
Chemical Formula:
|
|
C29H32O7S.1/2H2O
|
|
CAS No.:
|
|
1000413-72-8
|
|
Molecular Weight:
|
|
563.63
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
[(3S)-6-({2',6'-dimethyl-4'-[3-(methylsulfonyl)propoxy]biphe-nyl-3-yl}meth-oxy)-2,3-
dihydro-1-benzofuran-3-yl]acetic acid hemi-hydrate
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
TAK-875, TAK875
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
References:
1. Negoro et al., ACS Med. Chem. Lett. 2010, 1, 290-294.
2. Tsujihata et al., TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1
agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting
hyperglycemia in type 2 diabetic rats. J. Pharmacol. Exp. Ther. 2011, 339(1), 228-237.
|
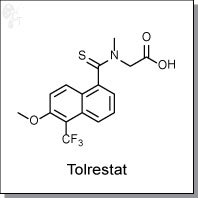
Product Name: Tolrestat | Aldose reductase inhibitor
|
|
|
Tolrestat is a thioamide-based inhibitor of aldose reductase with an IC50 activity of 35 nM. Along with other
similar therapies, Tolrestat has implications in the treatment of diabetic complications, such as diabetic
neuropathy, in a manner independent of insulin-related control of blood glucose levels.
In rat diabetes models, Tolrestat reduces sorbitol concentrations in the sciatic nerve and eye lens, as well
as prevents diabetic retinopathy, cateracts, thickening of the retinal basement membrane, and nerve
dysfunction. Additionally, Tolrestat reduces urinary albumin excretion.
|
Details
|
Chemical Formula:
|
|
C16H14F3NO3S
|
|
CAS No.:
|
|
82964-04-3
|
|
Molecular Weight:
|
|
357.35
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
2-(6-methoxy-N-methyl-5-(trifluoromethyl)naphthalene-1-carbothioamido)acetic acid
|
|
Solubility:
|
|
Up to 22 mM in DMSO
|
|
Synonyms:
|
|
Tolrestat
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
References:
1. Sestanj et al., N-[[5 -(Trifluoromethyl)-6-methoxy-1-naphthalenyl]thioxomethyl]-N-methylglycine
(Tolrestat), a Potent, Orally Active Aldose Reductase Inhibitor. J. Med. Chem. 1984, 27, 255-256.
2. Kador et al., Aldose Reductase Inhibitors: A Potential New Class of Agents for the Pharmacological Control
of Certain Diabetic Complications. J. Med. Chem. 1985, 28(7), 841-849.
3. Fruncillo et al., Pharmacokinetics of the aldose reductase inhibitor tolrestat: Studies in healthy young and
elderly male and female subjects and in subjects with diabetes. Clin. Pharmcol. Ther. 1996, 59(6), 602-613.
|
|


