|
Disease Area, Cardiovascular & Metabolic III
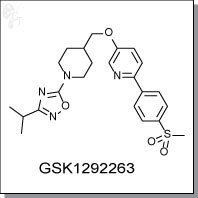
Product Name: GSK1292263 | GPR119 agonist
|
|
|
GSK1292263 is a novel GPR119 receptor agonist used for the treatment of type 2 diabetes. The predicted EC50
of GSK1292263 against GPR119 in vitro is at the nanomolar range. (1) The GPR119 receptor is a GPCR which
is selectively expressed on pancreatic beta cells and intestinal enteroendocrine cells. Agonists to GPR119
stimulate glucose-dependent insulin secretion in vitro and lower an elevated blood glucose level in vivo.(2)
In vivo, GSK1292263 treatment significantly increases in the peak insulin response and insulin AUC(0-15 min)
by 30-60% compared with vehicle control in the intravenous glucose tolerance test. This insulin upregulation
correlated with a significant increase in the glucose disposal rate.
|
Details
|
Chemical Formula:
|
|
C23H28N4O4S
|
|
CAS No.:
|
|
1032823-75-8
|
|
Molecular Weight:
|
|
456.56
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
3-isopropyl-5-(4-(((6-(4-(methylsulfonyl)phenyl)pyridin-3-yl)oxy)methyl)piperidin-
1-yl)-1,2,4-oxadiazole
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
GSK1292263, GSK 1292263, GSK-1292263
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Zhu X et al., The first pharmacophore model for potent G protein-coupled receptor 119 agonist. Eur J Med
Chem. 2011, 46(7):2901-7
2. Overton HA, et al., GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes
and obesity. Br J Pharmacol. 2008, 153 Suppl 1:S76-81.
3. Brown, K.K. et al. Diabetes [70th Annu Meet Sci Sess Am Diabetes Assoc (ADA) (June 25-29, Orlando)
2010] 2010, 59(Suppl. 1): Abst 407
|
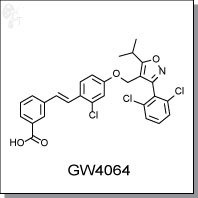
Product Name: GW4064 | non-steroidal FXR agonist
|
|
|
GW501516 is a selective PPARd agonist with a Ki affinity of 1.1 nM in a human binding assay. In a cell-based
transfection assay, GW501516 induces expression of GAL4-responsive reporter gene at an EC50 of 1.2 nM. At
doses up to 1 uM, GW501516 does not promote adipocyte differentiation, bind to RXRa, or have activity toward
other nuclear or non-nuclear receptors. (1) GW501516 incrases expression of the reverse cholesterol
transporter ATP-binding cassette A1 and induces apolipoprotein A1-specific cholesterol efflux. (1)
GW501516 is also reported to be a PPARb agonist with anti-inflammatory activity and decreases IFN-g-
induced upregulation of TNF-a and iNOS. Concurrently, GW501516 increase IL-6 mRNA expression. (2)
|
Details
|
Chemical Formula:
|
|
C21H18F3NO3S2
|
|
CAS No.:
|
|
317318-70-0
|
|
Molecular Weight:
|
|
453.5
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
2-(2-methyl-4-((4-methyl-2-(4-(trifluoromethyl)phenyl)thiazol-5-yl)methylthio)phenoxy)
acetic acid
|
|
Synoyms:
|
|
GW501516, GW-501,516, GW1516, GSK-516, Endurobol
|
|
Solubility:
|
|
Up to 22 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Oliver et al., A selective peroxisome proliferator-activated receptor d agonist promotes reverse cholesterol
transport. Proc. Nat'l. Acad. Sci. 2001, 98(9), 5306-5311.
2. Defaux et al., Effects of the PPAR-b agonist GW501516 in an in vitro model of brain inflammation and
antibody-induced demyelination. J. Neuroinflammation, 2009, 6, 15-27.
|
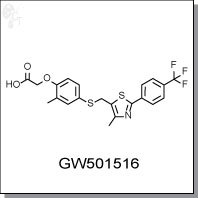
Product Name: GW501516 | PPARδ agonist
|
|
|
GW501516 is a selective PPARd agonist with a Ki affinity of 1.1 nM in a human binding assay. In a cell-based
transfection assay, GW501516 induces expression of GAL4-responsive reporter gene at an EC50 of 1.2 nM. At
doses up to 1 uM, GW501516 does not promote adipocyte differentiation, bind to RXRa, or have activity toward
other nuclear or non-nuclear receptors. (1) GW501516 incrases expression of the reverse cholesterol
transporter ATP-binding cassette A1 and induces apolipoprotein A1-specific cholesterol efflux.
GW501516 is also reported to be a PPARb agonist with anti-inflammatory activity and decreases IFN-g-
induced upregulation of TNF-a and iNOS. Concurrently, GW501516 increase IL-6 mRNA expression.
|
Details
|
Chemical Formula:
|
|
C21H18F3NO3S2
|
|
CAS No.:
|
|
317318-70-0
|
|
Molecular Weight:
|
|
453.5
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
2-(2-methyl-4-((4-methyl-2-(4-(trifluoromethyl)phenyl)thiazol-5-yl)methylthio)
phenoxy)acetic acid
|
|
Solubility:
|
|
Up to 22 mM in DMSO
|
|
Synonyms:
|
|
GW501516, GW-501,516, GW1516, GSK-516, Endurobol
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Oliver et al., A selective peroxisome proliferator-activated receptor d agonist promotes reverse cholesterol
transport. Proc. Nat'l. Acad. Sci. 2001, 98(9), 5306-5311.
2. Defaux et al., Effects of the PPAR-b agonist GW501516 in an in vitro model of brain inflammation and
antibody-induced demyelination. J. Neuroinflammation, 2009, 6, 15-27.
|
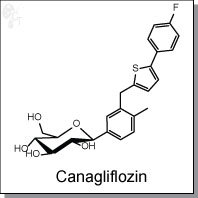
Product Name: JNJ-28431754 (Canagliflozin) | SGLT2 inhibitor
|
|
|
Canagliflozin is an orally-available, C-aryl glucoside inhibitor of hSGLT2, rSGLT2, and mSGLT2 cells at a
potency of 4.4, 3.7, and 2.0 nM, respectively. [1] It inhibits AMG uptake in CHO-hSGLT1 cells with an IC50 of
684 nM. Canagliflozin lowers renal glucose resorptive capacity and increases urinary glucose excretion.
Additionally, Canagliflozin has been shown to improve beta-cell function in ZDF rats, reduce body weight,
increase fatty acid oxidation, and reduce de novo lipgenesisin rodent models of insulin resistance and Type
II diabetes mellitus. [1]
In a twelve week study, Canagliflozin reduces HbA1c slightly more than sitagliptin (-0.21%) in a dose-
dependent manner. [2] Reductions in blood pressure have also been observed in similar studies.
Canagliflozin was approved by the FDA for the treatment of Type 2 diabetes in January, 2013.
|
Details
|
Chemical Formula:
|
|
C24H25FO5S
|
|
CAS No.:
|
|
842133-18-0
|
|
Molecular Weight:
|
|
444.52
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
(2S,3R,4R,5S,6R)-2-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-6-
(hydroxymethyl)-tetrahydro-2H-pyran-3,4,5-triol
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
JNJ-28431754 , JNJ 28431754, TA-7284, Canagliflozin, Invokana
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Liang et al., Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal
and diabetic animal models. PLoS ONE, 2012, 7(2), e30555.
2. Clar et al., Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ
Open, 2012, 2, e001007.
3. Nomura et al., Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent
glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J. Med. Chem. 2010, 53, 6355-
6360.
|
|



