|
Disease Area, Cardiovascular & Metabolic I
Product Name: A769662 l AMPK activator
|
|
|
A769662 is a potent, reversible AMP-activated protein kinase (AMPK) activator. It activates AMPK through the
β subunit carbohydrate-binding module and the γ subunit but not the AMP-binding sites.
A769662 stimulated partially purified rat liver AMPK with an EC50 of 0.8 µM and inhibited fatty acid synthesis
in primary rat hepatocytes with an IC50 of 3.2 µM. A769662 could be a useful tool compound for both cell
survival and cell proliferation.
|
Details
|
Chemical Formula:
|
|
C20H12N2O3S
|
|
CAS No.:
|
|
844499-71-4
|
|
Molecular Weight:
|
|
360.39
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid or off white solid
|
|
Chemical Name:
|
|
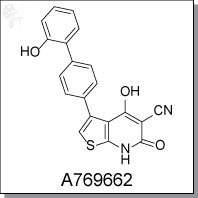
4-hydroxy-3-(2'-hydroxy-[1,1'-biphenyl]-4-yl)-6-oxo-6,7-dihydrothieno[2,3-b]
pyridine-5-carbonitrile
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Scott, J.W., et al. Thienopyridone drugs are selective activators of AMP-activated protein kinase β1-
containing complexes. Chem. Biol. 15: 1220-1230 (2008).
2. Cool, B., et al. Identification and characterization of a small molecule AMPK activator that treats key
components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3: 403-416 (2006).
3. Huang, X. et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient
mice. Biochem J. 412:211-221 (2008).
|
Product Name: AICAR | AMPK activator
|
|
|
AICAR is a natural metabolic intermediate of the purine biosynthetic pathway. In its monophosphate form,
AICAR is a potent AMPK agonist, which at low concentrations, does not affect AMP, ADP, or ATP levels.
AICAR's interacts directly with AMPK, inducing a conformational change that favors phosphorylation of the
catalytic alpha subunit.
AMPK is also implicated in the modulation of enzymes such as glucokinase and glycogen phosphorylase.
Direct binding by AICAR to phosphofructokinase and fructose-1,6-bisphosphatase have been reported.
AICAR's interaction with HSP90 has also been studied and found to have antiproliferative effects in tumor cell
lines such as PC-3, MCF-7, C6 glioma, U87MG, K-562, and CEM.
|
Details
|
Chemical Formula:
|
|
C9H14N4O5
|
|
CAS No.:
|
|
2627-69-2
|
|
Molecular Weight:
|
|
258.23
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
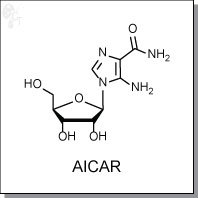
5-amino-1-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1H-
imidazole-4-carboxamide
|
|
Solubility:
|
|
Up to 22 mM in DMSO
|
|
Synonyms:
|
|
Arasine, AICA-riboside, Protara, AIC-Riboside
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Daignan-Fornier et al., 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranosyl 5'-Monophosphate
(AICAR), a Highly Conserved Purine Intermediate with Multiple Effects Metabolites 2012, 2, 292-302.
2.Guo et al., The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting
lipogenesis. Proc. Natl. Acad. Sci. 2009, 106(31), 12932-12937.
|
Product Name: Aliskiren | direct renin inhibitor
|
|
|
Aliskiren is an orally-available, nonpeptide piperidine-based inhibitor renin (IC50 0.6 nM) for the treatment of
hypertension. Despite it's 2.5% bioavailability in humans, the absorbed aliskiren is slowly cleared such that
the drug can accumulate to therapeutic levels within several doses.
Aliskiren is highly selective for the Renin-Angiotensin System (RAS) and therefore does not interfere with
bradykinin metabolism; therefore side effects often associated with ACE inhibitors are not likely to occur with
aliskiren. Aliskiren is effective in reducing blood pressure and is well tolerated, and has shown promising
target organ protection in animal models and clinical trials.
|
Details
|
Chemical Formula:
|
|
C30H53N3O6
|
|
CAS No.:
|
|
173334-57-1, 173334-58-2
|
|
Molecular Weight:
|
|
551.76
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
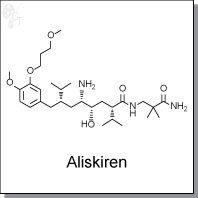
(2S,4S,5S,7S)-5-Amino-N-(2-carbamoyl-2-methylpropyl)-4-hydroxy-2-isopropyl-7-
[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methylnonanamide
|
|
Solubility:
|
|
Up to 22 mM in DMSO
|
|
Synonyms:
|
|
Aliskiren, Rasilez, Tekturna, CGP60536B
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Brown, M.J., Aliskiren. Circulation, 2008, 118, 773-784.
2. Allikmets, K., Aliskiren--an orally active renin inhibitor. Review of pharmacology, pharmacodynamics,
kinetics, and clinical potential in the treatment of hypertension.Vasc. Heatlh Risk Mgmt., 2007, 3(6), 809-815.
3. Gradman et al., Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive
efficacy and placebo-like tolerability in hypertensive patients. Circulation 2005, 111, 1012-1018. Pubmed ID:
15723979
|
Product Name: BI-1356 (Linagliptin) | DPP-IV/DPP-4 inhibitor
|
|
|
BI-1356 (Linagliptin) is a highly potent and selective dipeptidyl peptidase 4 (DPP-4) inhibitor (IC50 = 1 nM) for
treatment of type II diabetes. BI-1356 can increase incretin levels (GLP-1 and GIP), which increases insulin
secretion and inhibits glucagon release, decreases gastric emptying, and decreases blood glucose levels.
BI-1356 shows 10,000-fold more selectivity for DPP-4 against other protease/peptidases, including DPP-8,
DPP-9, trypsin, plasmin, and thrombin.
|
Details
|
Chemical Formula:
|
|
C25H28N8O2
|
|
CAS No.:
|
|
668270-12-0
|
|
Molecular Weight:
|
|
472.54
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
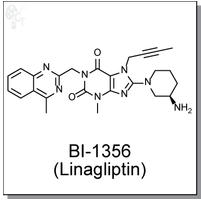
(R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-methyl-1-((4-methylquinazolin-2-yl)
methyl)-1H-purine-2,6(3H,7H)-dione
|
|
Solubility:
|
|
Up to 25 mM in DMSO
|
|
Synonyms:
|
|
BI-1356, BI1356, Linagliptin, Tradjenta, Trajenta
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Eckhardt M, et al. 8-(3-(R)-aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-
ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a highly potent, selective, long-acting, and orally
bioavailable DPP-4 inhibitor for the treatment of type 2 diabetes. J Med Chem. 2007; 50(26):6450-3.
2. Thomas L, et al. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-
ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor,
has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J
Pharmacol Exp Ther. 2008; 325(1):175-82.
|
|


