Enhanced generic CHO|360-HCP ELISA kit
본문
|
Enhanced generic CHO|360-HCP (Host Cell Protein) ELISA kit
360-HCP(Host Cell Protein) ELISA 키트는 기존의 HCP 분석에서 문제가 되고 있던, 샘플 중 HCP가 완전히 검출되지 않은 단점을 보완한 최신의 제품입니다. 기존의 generic HCP 분석은 모든 과정을 단 한 종류의 항체로 하지만, "Enhanced generic 360-HCP ELISA"에서는 4 종류의 키트 (각각 다른 anti-HCP 항체를 사용)를 제공합니다. 여러 항체를 사용하여서 샘플에 가장 적합한 항체 를 찾을 수 있습니다.
The 360-HCP (Host Cell Protein) ELISA Approach The enhanced generic 360-HCP approach produces host cell protein (HCP) assays that are demonstrably superior to traditional generic HCP assays minimizing well-known limitations including the possibility that anti-HCP antibodies might not comprehensively detect HCPs present in an end-user's sample. While traditional generic HCP assays provide just a single antibody that must work for all processes the enhanced generic 360-HCP ELISA provides four kits, each using a different anti-HCP antibody so you can try multiple antibodies to find the one that works best for your samples.
This unique approach increases the assay specificity and sensitivity and allows sponsors and biotech companies to postpone the development of a cost-intensive specific HCP assay until a more informed decision on the success of a biologic in development can be made.
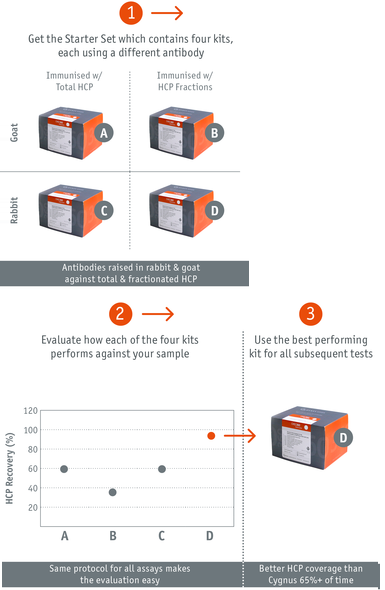
How it works
How we made the antibodies Polyclonal HCP antiserum was generated by immunizing rabbits and goats with HCP derived from mock transfected CHO-K1 and CHO-S cells combined with a unique approach for antigen and antibody preparation as well as an optimized purification strategy. We used differently prepared antigens: total HCP or fractionated HCP. Each HCP fraction was used individually for immunization resulting in four differentHCP ELISA kits (type A to D) that together build up the enhanced generic CHO|360-HCP assay.
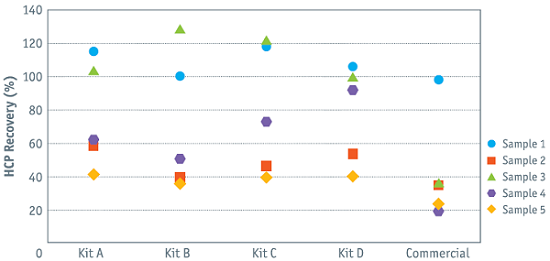
Superior HCP Recovery The CHO|360-HCP ELISA has been widely tested on the basis of a great number of mock CHO-HCP samples. In most cases, the best recovery was estimated with one of our four generic CHO-HCP assays (type A to D). In the case of sampel 4(x), a recovery of >90% was estimated using assay type D. Recovery with assay types A to C was much lower, and recovery with the commercial assay was only 20%.
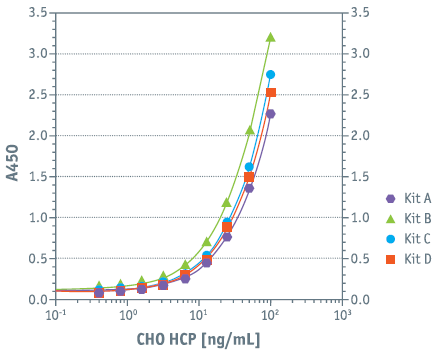
Product Specifications Sensitivity CHO|360-HCP ELISA Kits (A–D)
• LOD between 0.5 – 1.0 ng/mL • LOQ between 2 – 3 ng/mL • Working range 2 – 100 ng/mL
Instruction sheet (PDF) Qualification report(PDF) |
Ordering informations
Starter set
|
Catalog No. |
Product name |
Size |
|
IP 001 |
CHO|360-HCP ELISA, Type A-D starter set |
1 x type A, 1 x type B, 1 x type C & 1 x type D |
CHO|360-HCP ELISA kit
|
Catalog No. |
Product name |
Size |
|
IP 101 |
CHO 360 HCP ELISA Type A |
kit |
|
IP 002 |
CHO 360 HCP ELISA Type B |
kit |
|
IP 003 |
CHO 360 HCP ELISA Type C |
kti |
|
IP 004 |
CHO 360 HCP ELISA Type D |
kit |
Antibody / Standard / Assay buffer
|
Catalog No. |
Product name |
Size |
|
IP 111 |
Anti CHO HCP antibody, Type A |
0.5 mg |
|
IP 112 |
Anti CHO HCP antibody, Type B |
0.5 mg |
|
IP 113 |
Anti CHO HCP antibody, Type C |
0.5 mg |
|
IP 114 |
Anti CHO HCP antibody, Type D |
0.5 mg |
|
IP 120 |
CHO HCP standard |
0.5 ml |
|
IP 130 |
Assay buffer |
100 ml |
▣ 관련 페이지 ; BioGenes GmbH
댓글목록
등록된 댓글이 없습니다.