TMP195 & other new product of Cellagen Technology
본문
TMP195 & other new product of Cellagen Technology
Product Name: TMP 195
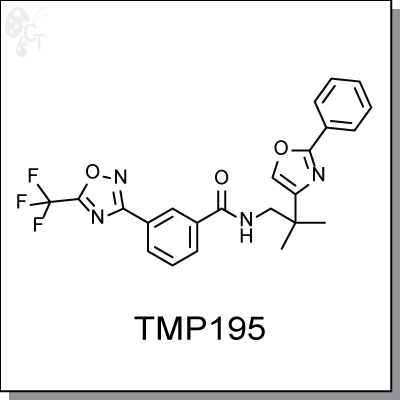
TMP195 is the most potent and selective class IIa HDAC inhibitor identified to date, with IC50s of 59 nM, 60 nM, 26 nM
and 15 nM for HDAC4, HDAC5, HDAC7 and HDAC9 respectively. It has over 100-fold selectivity targeting other HDACs
(IC50s>10 µM). TMP195 has an unprecedented zinc-binding group, trifluoromethyloxadiazole (TFMO), which circumvents the selectivity and pharmacologic liabilities of hydroxamates. (1)
TMP195 alters the gene expression of monocytes upon treatment of colony-stimulating factors, while showing no
cytotoxicity towards T cell, B cells and monocytes. This tool compound help revealed the unique cellular activities of class IIa HDACs. (1) The discovery of TMP195 and related compounds provides an alternative design for targeting
metalloenzymes than the conventional chelating metal-binding group, and suggests a therapeutic potential for class IIa
HDAC inhibitors distinct in mechanism and application compared to current HDAC inhibitors (e.g. Trichostatin A, or TSA).
Details
|
Chemical Formula: |
|
C23H19F3N4O3 |
|
CAS No.: |
|
N/A |
|
Molecular weight: |
|
456.42 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White |
|
Chemical name: |
|
N-(2-methyl-2-(2-phenyloxazol-4-yl)propyl)-3-(5-(trifluoromethyl)
-1,2,4-oxadiazol-3-yl)benzamide |
|
Solubility: |
|
Up to 50 mM in DMSO |
|
Synonyms: |
|
TMP195 |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References:
1. Lobera M, et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. (2013) Nat
Chem Biol. 9(5):319-25. Pubmed ID: 23524983
Product Name: TMP 269
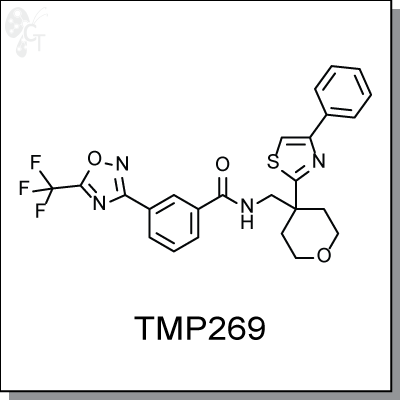
TMP269 is a highly potent and selective class IIa HDAC inhibitor identified, with IC50s of 126 nM, 80 nM, 36 nM and
19 nM for HDAC4, HDAC5, HDAC7 and HDAC9 respectively. TMP269 has an unprecedented metal-binding group,
trifluoromethyloxadiazole (TFMO). The co-crystal structure of TMP 269 and HDAC7 revealed that bulky TFMO and
U-shaped confirmation conferred potent and selective class IIa HDAC inhibition. [1]
In contrary to other HDAC inhibitors (TSA or SAHA), TMP269 showed no effect at the acetylation level of histone H3-
K9, no signifcant effects (2-fold) in gene expression on T-cells and no cytotoxicity in T cell expansion assay. [1] However,
inhibition of class IIa HDACs affected gene expression of CD14+ monocytes under PHA stimulation. This indicates the
unique cellular activities of class IIa HDACs. The discovery of TMP269 and related compounds provides an alternative
design for targeting metalloenzymes than the conventional chelating metal-binding group, and suggests a therapeutic
potential for class IIa HDAC inhibitors distinct in mechanism and application compared to traditional HDAC inhibitors (e.g.
TSA).
Details
|
Chemical Formula: |
|
C25H21F3N4O3S |
|
CAS No.: |
|
1314890-29-3 |
|
Molecular weight: |
|
514.52 |
|
Purity: |
|
> 99% |
|
Appearance: |
|
White |
|
Chemical name: |
|
N-((4-(4-phenylthiazol-2-yl)tetrahydro-2H-pyran-4-yl)methyl)-3-(5-(trifluoromethyl)
-1,2,4-oxadiazol-3-yl)benzamide |
|
Solubility: |
|
Up to 50 mM in DMSO |
|
Synonyms: |
|
TMP269 |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
Reference
1. Lobera M, et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. (2013) Nat
Chem Biol. 9(5):319-25. Pubmed ID: 23524983
Ordering informations
|
Catalog No. |
Product Name |
Size |
|
C8619-2 |
TMP 195 / Class IIa HDAC inhibitor |
2mg, 10mg & 50mg |
|
C8626-2 |
TMP 269 / Class IIa HDAC inhibitor |
2mg, 10mg & 50mg |
* 관련제품 정보
▣ 관련 페이지 ; Cellangen Technology
댓글목록
등록된 댓글이 없습니다.