Inhibitors related stem cell from Cellagen (4)
본문
Inhibitors related stem cell from Cellagen (4)
Product Name: SB431542 | TGF-β type I (ALK4/5/7) inhibitor (#C7243)
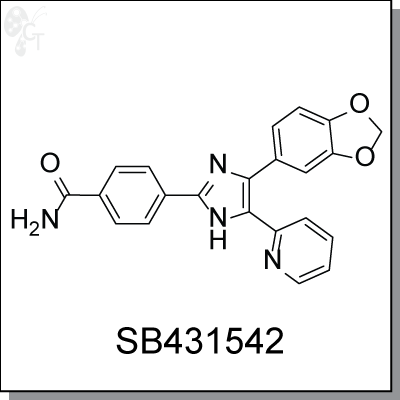
SB-431542 is a selective and potent inhibitor of activin receptor-like kinase (ALK, the TGF-β type I receptor), specifically
ALK4, ALK5 (IC50 = 94 nM) and ALK7. It has no effect on the other, more divergent ALK family members that recognize
bone morphogenetic proteins (BMPs) such as ALK2, ALK3 or ALK6. It has also no effect on components of the ERK, JNK,
or p38 MAP kinase pathways or on components of the signaling. SB431542 specifically blocks Smad signaling and
suppresses renewal in embryonic and induced pluripotent stem (iPS) cells and promotes differentiation. SB431542 could
enhance reprogramming efficiency when using it with MEK inhibitor PD0325901.
Details
|
Chemical Formula: |
|
C22H16N4O3 |
|
CAS No.: |
|
301836-41-9 |
|
Molecular weight: |
|
384.39 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
Off white solid |
|
Chemical name: |
|
4-(4-(benzo[d][1,3]dioxol-5-yl)-5-(pyridin-2-yl)-1H-imidazol-2-yl)benzamide |
|
Solubility: |
|
Up to 100 mM in DMSO |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References
1. Tongxiang Lin, et al. A chemical platform for improved induction of human iPSCs. Nature Methods 6, 805 - 808 (2009).
2. Laping NJ, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor
of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002; 62(1):58-64.
3. Inman GJ, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I
Product Name: Spautin-1 | Autophagy inhibitor (#C3430)
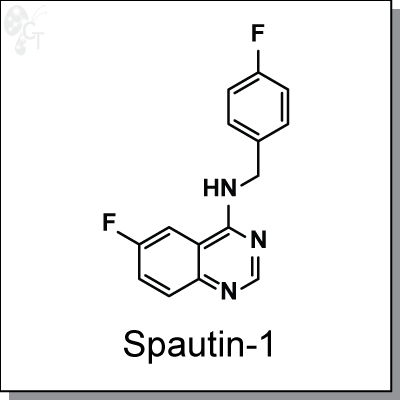
Spautin-1 is a specific and potent autophagy inhibitor (IC50=0.74µM). It promotes the degradation of Vps34 PI3 kinase
complexes by inhibiting two ubiquitin specific peptidases, USP10 and USP13, that target the Beclin1 subunit of Vps34
complexes. Since USP10 mediates the deubiquitination of p53, regulating deubiquitination activity of USP10 and USP13 by
Beclin1 provides a mechanism for Beclin1 to control the levels of p53. By this mechanism, Spautin-1 increased cancer cell
death in the setting of nutrient deprivation when autophagy would normally act as a survival mechanism in these
metabolically stressed cells.
Details
|
Chemical Formula: |
|
C15H11F2N3 |
|
CAS No.: |
|
1262888-28-7 |
|
Molecular weight: |
|
271.26 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White solid |
|
Chemical name: |
|
6-fluoro-N-(4-fluorobenzyl)quinazolin-4-amine |
|
Solubility: |
|
Up to 100 mM in DMSO |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References
1. Junli Liu, et al. Beclin1 Controls the Levels of p53 by Regulating the Deubiquitination Activity of USP10 and USP13.
Cell (2011), 147(1), 223-234. .
2. Joseph D. Mancias, et al. Targeting Autophagy Addiction in Cancer. Oncotarget (2011). In press.
Product Name: Thiazovivin | ROCK inhibitor (#C8442)
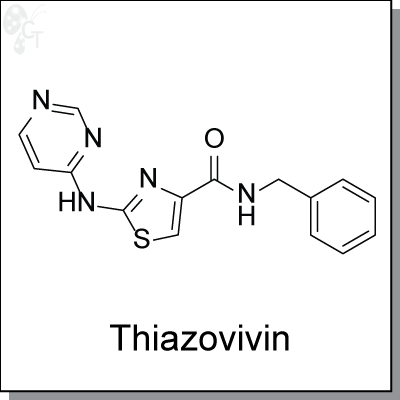
Thiazovivin is a potent ROCK inhibitor. It dramatically improves the survival of hESCs during cell trypsinization
manipulation. Thiazovivin in combination with inhibitors of the TGF-β receptor and the MEK pathway improve
reprogramming efficiency more than 200-fold.
Details
|
Chemical Formula: |
|
C15H13N5OS |
|
CAS No.: |
|
1226056-71-8 |
|
Molecular weight: |
|
311.36 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
Light brown solid |
|
Chemical name: |
|
N-Benzyl-2-(pyrimidin-4-ylamino)thiazole-4-carboxamide |
|
Solubility: |
|
Up to 100 mM in DMSO |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
Reference
1. Lin, T., Ambasudhan, R., Yuan, X., Li, W., Hilcove, S., Abujarour, R., Lin, X., Hahm, H., Hao, E., Hayek, A., Ding, S.
(2009) A chemical platform for improved induction of human iPSCs. Nature Methods 6, 805 - 808 (2009)
Product Name: Trichostatin A (TSA) | HDAC inhibitor (#C8742)
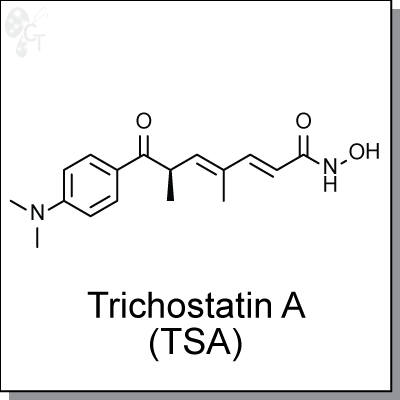
Trichostatin A (TSA) is a potent histone deacetylase (HDAC) inhibitor. It inhibits HDAC 1, 2, 3, 6, 10, 11 at IC50s of less than
10 nM, with over 300-fold selectivity against class IIa HDACs.[1] TSA affects DNA replication and gene expression by
inhibiting HDAC activity and therefore altering the histone modifications and access of DNA inside chromatin.
Trichostatin A induces apoptosis and cell growth arrest at both G and G/M phases. As HDACs are overexpressed in many
cancer types, TSA is widely used to probe the tumorigenesis mechanism targeting HDAC.[2] Trichostatin A was found to
prevent the differentiation of embryonic stem cell,[3] while TSA treatment increased functional characteristics of human
ESC/iPSC-derived cardiomyocytes.[4]
Details
|
Chemical Formula: |
|
C17H22N2O3 |
|
CAS No.: |
|
58880-19-6 |
|
Molecular weight: |
|
302.37 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
Brown |
|
Chemical name: |
|
(R,2E,4E)-6-(4-(dimethylamino)benzoyl)-N-hydroxy-4-methylhepta-2,4-dienamide |
|
Solubility: |
|
Up to 50 mM in DMSO |
|
Synonyms |
|
Trichostatin A, TSA |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References:
1. Lobera M, et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat Chem Biol.
2013; 9(5):319-25. Pubmed ID: 23524983
2. Timmermann S, et al. Histone acetylation and disease. Cell Mol Life Sci. 2001; 58(5-6):728-36. Review Pubmed
ID: 11437234
3. Lee JH, et al. Histone deacetylase activity is required for embryonic stem cell differentiation. Genesis. 2004; 38(1):32-8.
Pubmed ID: 14755802
4. Otsuji TG, et al. Dynamic link between histone H3 acetylation and an increase in the functional characteristics of human
ESC/iPSC-derived cardiomyocytes. PLoS One. 2012; 7(9):e45010 Pubmed ID: 11437234
Product Name: Wnt-C59 | Wnt signaling inhibitor (#C7641)
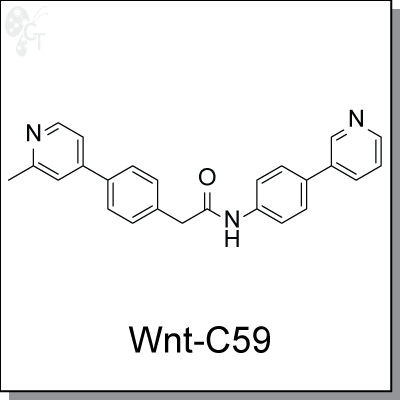
Wnt-C59 was first disclosed in patent WO2010101849 as a potent Wnt signaling modulator. It has IC50 less than 0.11 nM
in Wnt-Luc reporter assay for Wnt pathway inhibition. In the study, Wnt-C59 prevents palmitylation of Wnt proteins by
Porcupine (Porcn, a membrane-bound O-acyltransferase), thereby blocking Wnt secretion and activity, similar to Wnt
inhibitors IWP-2, IWP-3 and IWP-4. But Wnt-C59 is more potent and selective, and has better chemical/physical properties,
suitable for in vitro/in vivo studies.
Details
|
Chemical Formula: |
|
C25H21N3O |
|
CAS No.: |
|
1243243-89-1 |
|
Molecular weight: |
|
379.45 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
Pale yellow solid |
|
Chemical name: |
|
2-(4-(2-methylpyridin-4-yl)phenyl)-N-(4-(pyridin-3-yl)phenyl)acetamide |
|
Solubility: |
|
Up to 50 mM in DMSO |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References:
1. Dai Chen et al. (N-(HETERO)ARYL,2-(HETERO)ARYL-SUBSTITUTED ACETAMIDES FOR USE AS WNT SIGNALING
MODULATORS. PCT WO/2010/101849.
2. Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C,
Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer.
Nat Chem Biol. 2009;5(2):100-7.
Product Name: Y-27632 | ROCK inhibitor (#C9127)
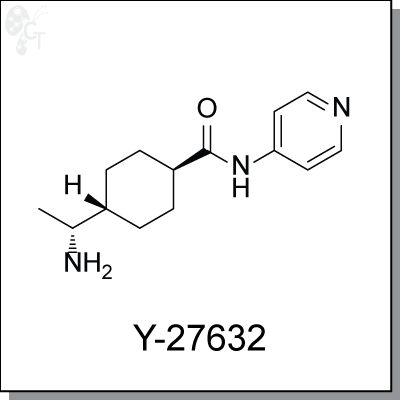
Y-27632 is widely used as a specific inhibitor of the Rho-associated coiled-coil forming protein serine/threonine kinase
(ROCK) family. It has been found to prevent dissociation-induced apoptosis in human embryonic stem cells and enhance
the survival and cloning efficiency of dissociated hES cells without affecting their self-renewal properties or pluripotency.
Y-27632 can also enhance survival during the transplantation of ES cell-derived neural precursors.
Details
|
Chemical Formula: |
|
C14H21N3O·2HCl |
|
CAS No.: |
|
146986-50-7 |
|
Molecular weight: |
|
329.27 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White solid |
|
Chemical name: |
|
(1R,4r)-4-((R)-1-aminoethyl)-N-(pyridin-4-yl)cyclohexanecarboxamide |
|
Solubility: |
|
Up to 100 mM in DMSO |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References
1. Ishizaki, T. et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol
(2000) 57: 976-983.
2. Koyanagi et al. Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem
cell-derived neural precursors. J. Neurosci Res (2008) 86: 270-280.
3. Watanabe et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotech (2007) 25:
681-686.
Ordering informations
|
Catalog No. |
Product Name |
Size |
|
C7243 |
SB431542 | TGF-β type I (ALK4/5/7) inhibitor |
5mg, 25mg & 100mg |
|
C3430 |
Spautin-1 | Autophagy inhibitor |
2mg, 10mg & 50mg |
|
C8442 |
NVP-XAV939 | Wnt/β-catenin Inhibitor |
1mg, 5mg & 25mg |
|
C8742 |
Trichostatin A (TSA) | HDAC inhibitor |
2mg, 10mg & 50mg |
|
C7641 |
Wnt-C59 | Wnt signaling inhibitor |
2mg, 10mg & 50mg |
|
C9127 |
Y-27632 | ROCK inhibitor |
2mg, 10mg & 50mg |
* 관련제품 정보
Stem Cell Pathway and Chemical Modulators
Stem Cell Pathway Modulating Compounds
▣ 관련 페이지 ; Cellangen Technology
댓글목록
등록된 댓글이 없습니다.