Cellular Mechanism, Angiogenesis
Product Name: BIBF1120 (Vargatef, Nintedanib) | VEGFR/PDGFR/FGFR inhibitor (#C2311)
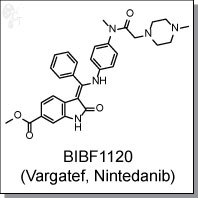
BIBF1120 (Vargatef, Nintedanib) is an oxindole-based, orally-available triple kinase inhibitor targeting the
angiogenesis factors VEGF, PDGF, and FGF. It inhibits VEGFR, PDGFR, and FGFR at potency ranges of 13-
34, 59-65, and 37-108 nM. [1] Kinase panel studies show that it also inhibits Flt-3, Lck, Lyn, and Src at 26,
16, 195, and 156 nM, respectively. BIBF1120 also inhibits cell proliferation in MAPK and Akt signaling
pathways at EC50s of 10-80 nM.
Continuous oral QD dosing of BIBF1120 displayed antitumor activity in a number of tumor cell lines (FaDu,
Caki-1, HT-29, SKOV-3, Calu-6, PAC-120, and GS-9L.
BIBF-1120 is being explored as a treatment for idiopathic pulmonary fibrosis and has shown promise in the
delay of lung fibrosis progression. [2]
|
Details
|
Chemical Formula:
|
|
C31H33N5O4
|
|
CAS No.:
|
|
928326-83-4
|
|
Molecular weight:
|
|
539.62
|
|
Purity:
|
|
> 99%
|
|
Appearance:
|
|
Yellow
|
|
Chemical name:
|
|
(Z)-methyl 3-((4-(N-methyl-2-(4-methylpiperazin-1-yl)acetamido)phenylamino)(phenyl)methylene)-2-oxoindoline-6-carboxylate
|
|
Solubility:
|
|
Up to 10 mM in DMSO
|
|
Synonyms:
|
|
BIBF-1120, BIBF 1120, BIBF1120, Vargatef, Nintedanib
|
|
Storage:
|
|
For longer shelf life, store solid powder or DMSO solution at -20oC
|
1. Hilberg et al., BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor
efficacy. Cancer Res. 2008, 68(12), 4774-4782. Pubmed ID: 18559524
2. Antoniu, S., Nintedanib (BIBF 1120) for IPF: a tomorrow therapy? Multidiscip. Respir. Med. 2012, 7, 41-45.
Pubmed ID: 23146151
|
Product Name: FG-4592 (ASP1517) | HIF prolyl-hydroxylase inhibitor (#C3445)
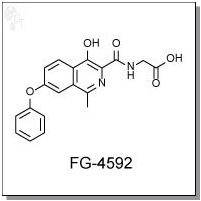
FG-4592 is an orally-available isoquinoline-based inhibitor of hypoxia-inducible factor (HIF) prolyl
hydroxylase for the treatment of anemia and patients with chronic kidney disease. [1] Phase 2 studies
indicate that oral administration of FG-4592 three times a week increased mean hemoglobin levels in the first
eight weeks, regardless of supplementation with IV or oral iron, or no iron supplementation. FG-4592 is novel
in that it allows integration of red blood cell production and efficient iron incorporation simultaneously. [1]
Data from Phase 2 trials comparing with epoetin alpha indicate that treatment with FG-4592 alone results in a
sustained reduction of total plasma cholesterol levels by an average of 20%, while no reduction was seen
with epoetin alpha. [2, 3]
In December, 2012, Fibrogen and Astellas Pharma announced the initiation of a Phase 3 clinical development
program for the treatment of anemia associated with chronic kidney disease in patients not on dialysis and
on dialysis. [4]
|
Details
|
Chemical Formula:
|
|
C19H16N2O5
|
|
CAS No.:
|
|
808118-40-3
|
|
Molecular weight:
|
|
352.34
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Yellow
|
|
Chemical name:
|
|
2-(4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carboxamido)acetic acid
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
FG-4592, FG4592, ASP1517
|
|
Storage:
|
|
For longer shelf life, store solid powder or DMSO solution at -20oC
|
References
1. "Correction of anemia without IV iron supplementation in incident dialysis patients" Fibrogen press release,
Nov. 5, 2012
2. "FG-4592 and epoetin alpha in Phase II anemia study", Datamonitor Research Store, Nov. 11, 1012.
3. "Hemoglobin correction and maintenance in end-stage renal patients" Fibrogen press release, Nov. 5, 2012
4. Fibrogen press release, Dec. 11, 2012 (Phase 3 announcement)
|
|

