Cellular Mechanism, Apoptosis II
Product Name: NVP-LBH589 (Panobinostat) | HDAC inhibitor (#C5245)
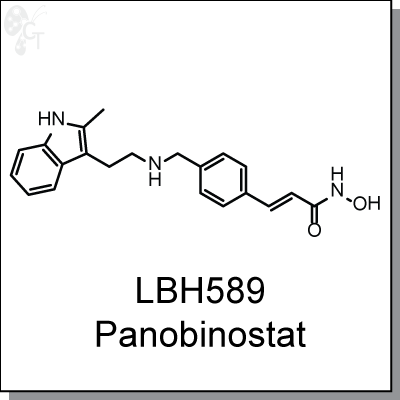
LBH589, a hydroxamate analog, is a broad-spectrum HDAC inhibitor. It has been shown to increase
acetylation of core histones (H3 and H4) and nonhistone proteins (alpha-tublin, HSP90), leading to the
modulation of gene expression (p21, FOXO3A, GADD45A, aromatase, etc) and protein activity involved in cell
growth and survival pathways1-4. LBH589 induces apoptosis in MOLT-4 and Reh cells with IC50 between 5
to 20nM1. In lung cancer and mesothelioma animal models, LBH589 markedly decreased tumor growth by
62% when compared with the vehicle control5. The anti-tumor activity of LBH589 has also been demonstrated
in many other cancer cell lines, including multiple myeloma, NSCLC and castrate-resistant prostate cancer
cell lines. LBH589 is under clinical trials to evaluate its effects in conjunction with chemotherapy and/or
targeted therapy in multiple cancer types.
|
Details
|
Chemical Formula:
|
|
C21H23N3O2
|
|
CAS No.:
|
|
404950-80-7
|
|
Molecular weight:
|
|
349.43
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical name:
|
|
(2E)-N-hydroxy-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]
acrylamide
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder or DMSO solution at -20oC
|
1. Scuto, A., et al. The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage
response genes and apoptosis in Ph- acute lymphoblastic leukemia cells. Blood. 2008 May 15;111(10)
:5093-100.
2. Chen, S. et al.The HDAC inhibitor LBH589 (panobinostat) is an inhibitory modulator of aromatase gene
expression. Proc Natl Acad Sci U S A 2010 Jun 15; 107(24) :11032-7.
3. George, P., et al. "Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG
is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3." Blood 105:
1768-1776 (2005).
4. Qian, D.Z., et al. "Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid
derivative LBH589." Clin. Cancer Res. 12: 634-642 (2006).
5. Crisanti, MC. et al. The HDAC inhibitor panobinostat (LBH589) inhibits mesothelioma and lung cancer cells
in vitro and in vivo with particular efficacy for small cell lung cancer. Mol Cancer Ther.
2009 Aug;8(8):2221-31.
|
Product Name: P7C3 | Neurogenic & neuroprotective agent (#C7723)
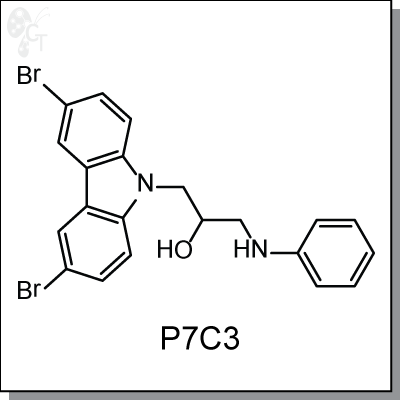
P7C3 is a proneurogenic, neuroprotective small molecule with favorable pharmacological properties
discovered from an in vivo screen. In vivo studies gave evidence that P7C3 exerts its proneurogenic activity
by protecting newborn neurons from apoptosis. Prolonged administration of P7C3 to npas3-/- mice corrected
their deficits by normalizing levels of apoptosis of newborn hippocampal neurons. Prolonged administration
of P7C3 to aged rats also enhanced neurogenesis in the dentate gyrus, impeded neuron death, and
preserved cognitive capacity as a function of terminal aging.
|
Details
|
Chemical Formula:
|
|
C21H18Br2N2O
|
|
CAS No.:
|
|
301353-96-8
|
|
Molecular weight:
|
|
474.19
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical name:
|
|
1-(3,6-dibromo-9H-carbazol-9-yl)-3-(phenylamino)propan-2-ol
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder or DMSO solution at -20oC
|
References
1. Andrew A Pieper, et al. Discovery of a proneurogenic, neuroprotective chemical. Cell (2010), 142(1), 39-51.
2. Karen S MacMillan, et al. Development of Proneurogenic, Neuroprotective Small Molecules. Journal of the
American Chemical Society (2011), 133(5), 1428-1437.
|
Product Name: S3I-201 (NSC74859) | Stat3 activity inhibitor (#C7341)
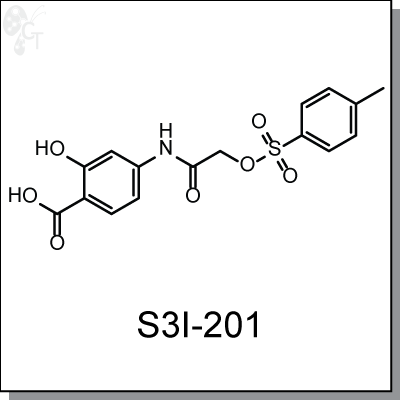
S3I-201 (NSC74859) is a cell-permeable, amidosalicylic acid based small molecule that inhibits Stat3 activity
with IC50 of 86 µM1. S3I-201 binds to the Stat3-SH2 domain and prevents its dimerization, inhibiting stat3
phosphorylation, translocation and Stat3-dependent transcription activities. S31-201 preferentially inhibits
growth and induces apoptosis in tumor cells that contain persistently activated stat31. In addition, S3I-201 has
been shown to retard stat3-dependent tumor growth in human breast tumor xenograft models, and to impair
VZV infection of skin xenografts in vivo1,2,3.
|
Details
|
Chemical Formula:
|
|
C16H15NO7S
|
|
CAS No.:
|
|
501919-59-1
|
|
Molecular weight:
|
|
365.36
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Off white solid
|
|
Chemical name:
|
|
2-Hydroxy-4-[[[(4-methylphenyl)sulfonyloxy]acetyl]amino]-benzoic acid
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder or DMSO solution at -20oC
|
References
1. Siddiquee K. et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual
screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007 May 1;104(18):7391-6.
2. Lin L, et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta
signaling. Oncogene, 2009, 28(7), 961-972.
3. Sen N, et al. Signal transducer and activator of transcription 3 (STAT3) and survivin induction by varicella-
zoster virus promote replication and skin pathogenesis. Proc Natl Acad Sci U S A, 2012, 109(2), 600-605.
|
Product Name: Staurosporine | pan-kinase inhibitor (#C7828)
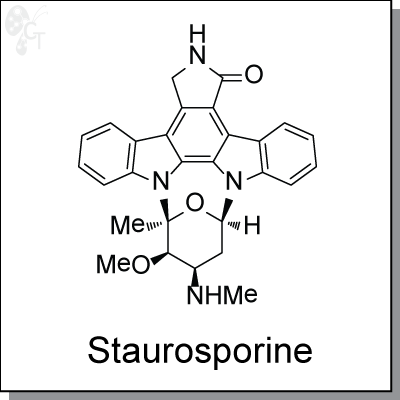
Staurosporine is a natural product isolated from the bacterium Streptomyces staurosporeus. It is a very
potent and broad spectrum protein kinase inhibitor through the prevention of ATP binding to the kinase. It
inhibits protein kinase C (IC50=0.7nM), CDK1/cyclin B (IC50=5nM), CDK2/cyclin A (IC50=7nM), CDK4/cyclin
D (IC50=3-10nM), CDK5/p25 (IC50=4nM), GSK-3β (IC50=15nM), Pim-1 kinase (IC50=10nM). It induces
apoptosis in human neuroblastoma cell lines and chick embryonic neurons. Due to its broad and potent
kinase inhibition activities, staurospoine is routinely used as cytotoxic, anti-proliferative reference compound.
|
Details
|
Chemical Formula:
|
|
C28H26N4O3
|
|
CAS No.:
|
|
62996-74-1
|
|
Molecular weight:
|
|
466.53
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical name:
|
|
(5S,6R,7R,9R)-6-methoxy-5-methyl-7-(methylamino)-6,7,8,9,15,16-hexahydro-17-
oxa-4b,9a,15-triaza-5,9-methanodibenzo[b,h]cyclonona[jkl]cyclopenta[e]-as-
indacen-14(5H)-one
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder or DMSO solution at -20oC
|
References
1. Tamaoki et al. Staurosporine, a potent inhibitor of phospholipid/Ca2+ dependent protein kinase. Biochem
Biophys Res Commun. (1986) 135 397.
2. Boix, J., et al. Characterization of the cell death process induced by staurosporine in human
neuroblastoma cell lines. Neuropharmacology (1997) 36: 811-821
3. Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. (2008) 26 (1):
127–132
|
Product Name: VX-765 | Caspase 1/Interleukin-converting enzyme inhibitor (#C8976)
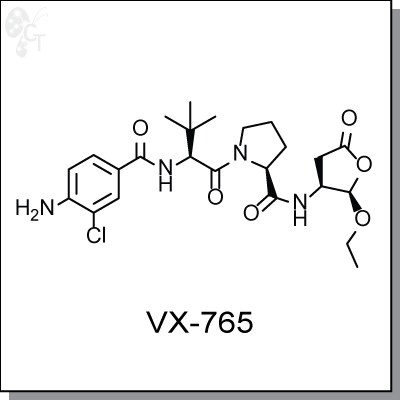
VX-765 is an orally-available prodrug of VRT-043198, an inhibitor of interleukin-converting enzyme /
caspase-1 subfamily caspases. VRT-043198 shows Ki values of 0.8 nM and 100-fold selectivity over other
non-ICE subfamily caspases. VRT-043198 showed no significant activity towards trypsin or cathepsin B. (1)
VRT-043198 inhibits IL-1b release from both PBMCs and whole blood with IC50 values of 0.67 and 1.9 uM,
respectively. Additionally in stimulated PBMCs, VRT-043198 dose-dependently inhibited IL-1b, IL-18, and
IFN-g, without affecting TNF-a release. In a hypoxia-induced apoptosis assay employing the NT2 human
neuroblastoma cell line, VRT-043198 did not alter ischemia-induced apoptosis up to concentrations of 100
uM. (1)
According to Vertex's news release , VX-765 has been shown to inhibit acute seizures in preclinical models
of acute epilepsy and has shown activity in preclinical models of chronic epilepsy that do not respond to
standard anti-epileptic drugs. (2)
|
Details
|
Chemical Formula:
|
|
C24H33CIN4O6
|
|
CAS No.:
|
|
273404-37-8
|
|
Molecular weight:
|
|
509
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
(S)-1-((S)-2-(4-amino-3-chlorobenzamido)-3,3-dimethylbutanoyl)-N-((2R,3S)-2-
ethoxy-5-oxotetrahydrofuran-3-yl)pyrrolidine-2-carboxamide
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Synonyms:
|
|
VX-765, VX765
|
|
Storage:
|
|
For longer shelf life, store solid powder or DMSO solution at -20oC
|
References
1. Wannamaker et al., (S)-1-((S)-2-{[1-(4-Amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-
butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765),
an Orally Available Selective Interleukin (IL)-Converting Enzyme/ Caspase-1 Inhibitor, Exhibits Potent Anti-
Inflammatory Activities by Inhibiting the Release of IL-1b and IL-18. J. Pharmacol. Exp. Ther. 2007, 321(2),
509-516. Pubmed ID: 17289835
2. http://investors.vrtx.com/releasedetail.cfm?ReleaseID=555967
|
|




