Cellular Mechanism, Metabolism_DNA/RNA synthesis III
Product Name: PSI-6206 (RO2433) l HCV RNA polymerase inhibitor (#C7620)
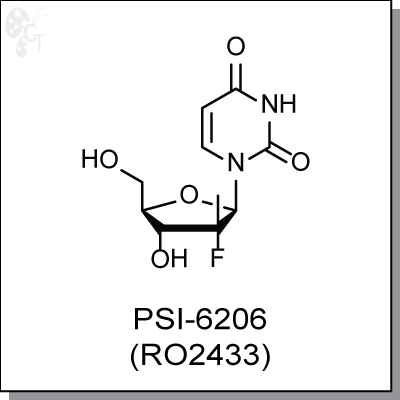
PSI-6206 (RO2433) is the unphosphorylated parent compound of triphosphate analog PSI-7409, which is a
potent inhibitor of the HCV NS5B RNA dependent RNA polymerase. The monophosphate form of PSI-6206
was shown to be metabolized in primary human hepatocytes to its triphosphate analog PSI-7409.
Furthermore, the phosphoramidate prodrug of PSI-6206 monophosphate, PSI-7851, was developed.
Alternatively, PSI-6130, an aminated analog of PSI-6206 monophosphate, was also developed. (1,2,3)
PSI-7409, the triphosphate of PSI-6206 inhibits wild-type and S282T HCV RdRp with Ki values of 0.42 and
22 uM, respectively. PSI-7851, the phosphoramidate of PSI-6206 monophosphate, showed an EC50 value of
1.62 uM for inhibiting HCV RNA replication. (2)
|
Details
|
Chemical Formula:
|
|
C10H13FN2O5
|
|
CAS No.:
|
|
863329-66-2
|
|
Molecular weight:
|
|
260.22
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Clear Crystal
|
|
Chemical name:
|
|
(2'R)-2'-Deoxy-2'-fluoro-2'-methyluridine
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
PSI-6206, RO-2433, PSI6206, RO2433
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution at -20oC
|
1. Rodriguez-Torres et al., Antiviral Activity, Pharmacokinetics, Safety, and Tolerability of PSI-7851, a Novel
Nucleotide Polymerase Inhibitor for HCV, Following Single and 3 Day Multiple Ascending Oral Doses in
Healthy Volunteers and Patients with Chronic HCV Infection. 60th AASLD Annual Meeting, 2009.
2. Murakami et al., The Mechanism of Action of b-D-2'-Deoxy-2'-Fluoro-2'-C-Methylcytidine Involves a
Second Metabolic Pathway Leading to b-D-2'-Deoxy-2'-Fluoro-2'-C-Methyluridine 5'-Triphosphate, a
Potent Inhibitor of the Hepatitis C Virus RNA-Dependent RNA Polymerase. Antimicrob. Agents. Chemother.
2008, 52(2), 458-464. Pubmed ID: 17999967
3. Ma et al., Characterization of the Metabolic Activation of Hepatitis C Virus Nucleoside Inhibitor b-D-2'-
Deoxy-2'-fluoro-2'-C-methylcytidine (PSI-6130) and Identification of a Novel Active 5'-Triphosphate
Species. J. Biol. Chem. 2007, 282, 29812-29820. Pubmed ID: 17698842
|
Product Name: PTC-124 (Ataluren) l Avoid early termination caused by nonsense
mutation (#C7124)
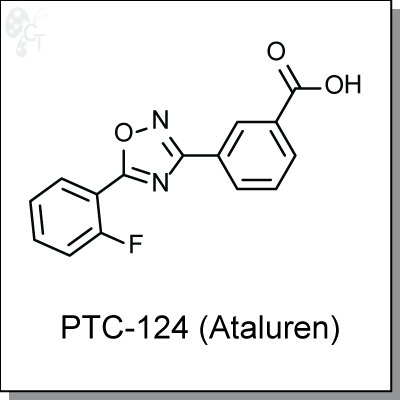
PTC-124 (Ataluren) is an oxadiazole-based, orally available agent deisgned to selectively promote ribosomal
readhtrough of premature stop codons, but not normal termination codons. [1] The minimal concentration of
PTC124 showing observable readthrough was 0.01 - 0.1 uM (2.8 - 28 ng/ml), while maximal activity was
observed at 3 uM (852 ng/ml). [2]
PTC-124 has been studied extensively in the treatment of cystic fibrosis and has shown good tolerability and
efficacy in preclinical and clinical settings. [3, 4]
|
Details
|
Chemical Formula:
|
|
C15H9FN2O3
|
|
CAS No.:
|
|
775304-57-9
|
|
Molecular weight:
|
|
284.24
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
3-(5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl)benzoic acid
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
PTC-124, PTC124, PTC 124
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution at -20oC
|
References
1. Sermet-Gaudelus et al., Ataluren (PTC124) induces cystic fibrosis transmembrane conductance regulator
protein expression and activity in children with nonsense mutation cystic fibrosis. Am. J. Respir. Crit. Care
Med. 2010, 182, 1262-1272. Pubmed ID: 20622033
2. Welch et al., PTC124 targets genetic disorders caused by nonsense mutations. Nature, 2007, 447, 87-91.
Pubmed ID: 17450125
3. Du et al., PTC124 is an orally bioavailable compound that promotes suppression of the human CFTR-
G542X nonsense allele in a CF mouse model. Proc. Natl. Acad. Sci. 2008, 105(6), 2064-2069
Pubmed ID: 18272502
4. Wilschanski et al., Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur. Respir.
J. 2011, 38, 59-69. Pubmed ID: 17450125
|
Product Name: TMC125 (Etravirine) | HIV-1 reverse transcriptase inhibitor (#C8125)
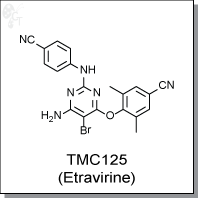
TMC125 is a nonnucleoside reverse transcriptase inhibitor with an EC50 of 1.4 to 4.3 nM for wild-type HIV-1.
Additionally, TMC125 is highly potent against a wide range of single-mutant and double-mutant NNRTI-
resistant HIV-1 strains, including L100I, K103N, Y181C, and L100I+K103N, at EC50s of 3, 1, 7, and 19 nM,
respectively. (1)
TMC125 is believed to have a high barrier to development of resistance in vitro based on multiplicity of
infection experiments which confirm a profile distinct from other reverse transcriptase inhibitors. (2)
|
Details
|
Chemical Formula:
|
|
C20H15BrN6O
|
|
CAS No.:
|
|
269055-15-4
|
|
Molecular weight:
|
|
435.28
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
white
|
|
Chemical name:
|
|
4-((6-amino-5-bromo-2-((4-cyanophenyl)amino)pyrimidin-4-yl)oxy)-3,5-
dimethylbenzonitrile
|
|
Solubility:
|
|
Up to 50 mM in DMSO
|
|
Synonyms:
|
|
TMC125, TMC-125, Etravirine
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution at -20oC
|
References
1. Andries et al., TMC125, a Novel Next-Generation Nonnucleoside Reverse Transcriptase Inhibitor Active
against Nonnucleoside Reverse Transcriptase Inhibitor-Resistant Human Immunodeficiency Virus Type 1.
Antimicrob. Agents Chemother. 2004, 48(12), 4680-4686. Pubmed ID: 15561844
2. Vingerhoets et al., TMC125 Displays a High Genetic Barrier to the Development of Resistance: Evidence
from In Vitro Selection Experiments. J. Virol. 2005, 79(20), 12773-12782. Pubmed ID: 16188980
|
|


