Cellular Mechanism, Metabolism_DNA/RNA synthesis II
Product Name: CX-5461 l RNA Pol l inhibitor (#C2954)
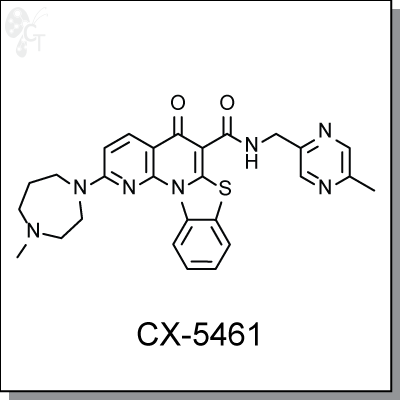
CX-5461 is a potent, orally bioavailable small molecule inhibitor of rRNA synthesis in cancer cells. It
selectively inhibits rRNA synthesis by polymerase I (Pol I) in the nucleolus, but does not inhibit mRNA
synthesis by RNA Polymerase II (Pol II), DNA replication or protein synthesis. (In tested cell lines, IC50s for
Pol I range from 54nmol/L to 142nmol/L, IC50s for Pol II is higher than 25umol/L.)
CX-5461 exhibits a broad range of antiproliferative activity, with wild-type (wt) p53 cells derived from
hematological malignancies being the most sensitive. (p53 wt solid tumors: median IC50=164 nM; p53 wt
hematologic cancers cells: median IC50=25 nM). CX-5461 selectively kills cancer cells relative to normal
cells. (Median IC50 in normal cells is 5,000 nM.) CX-5461 directly targets the initiation stage of rRNA
synthesis, induces both autophagy and senescene, but not apoptosis in a p53-independent process in solid
tumor cell lines. In wt p53 hematologic cancer cells, however, inhibition of Pol I results in nucleolar stress
and release of ribosomal proteins (RP) from the nucleolus. The RP bind to Mdm2 and release p53 to cause
apoptosis in cancer cells. CX-5461 exhibits potent in vivo antitumor activity against both human solid tumor in
xenograft models, and leukemia and lymphoma in animal models [1-2].
|
Details
|
Chemical Formula:
|
|
C27H27N7O2S
|
|
CAS No.:
|
|
1138549-39-6
|
|
Molecular weight:
|
|
513.61
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
5H-Benzothiazolo[3,2-a][1,8]naphthyridine-6-carboxamide, 2-(hexahydro-4-methyl-1H-1,4-diazepin-1-yl)-N-[(5-methyl-2-pyrazinyl)methyl]-5-oxo-
|
|
Solubility:
|
|
Up to 1 mM in DMSO
|
|
Synonyms:
|
|
CX-5461, CX5461
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution at -20oC
|
1. Drygin D., et al. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA
synthesis and solid tumor growth. Cancer Res. 2011. 71(4):1418-30. Pubmed ID: 21159662
2. Bywater MJ., et al. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific
activation of p53. Cancer Cell. 2012. 22(1):51-65. Pubmed ID: 22789538
|
Product Name: Daunorubicin hydrochloride l DNA intercating agent (#C3286)
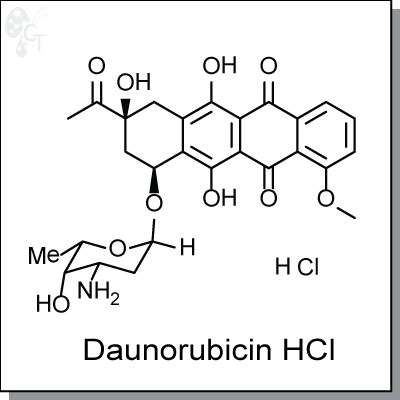
Daunorubicin is an anthracycline drug used that killing cells by inhibiting DNA and RNA synthesis. It
intercalates between base pairs and interacts with topoisomerase II, therefore inhibiting DNA replication and
repair and RNA synthesis. Daunorubicin was initially isolated from Streptomyces peucetius.
In vitro, daunorubicin inhibits the proliferation of various tumor cells at micromolar level. (1) Clinically, it has
been used in treating certain types of acute leukemia and neuroblastoma.
|
Details
|
Chemical Formula:
|
|
C27H29NO10HCl
|
|
CAS No.:
|
|
23541-50-6
|
|
Molecular weight:
|
|
563.98
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Orange RED
|
|
Chemical name:
|
|
(8S,10S)-8-acetyl-10-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione, monohydrochloride
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
Daunorubicin hydrochloride
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution at -20oC
|
Reference
1. Gewirtz DA, A critical evaluation of the mechanisms of action proposed for the antitumor effects of the
anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727-41.
Pubmed ID: 10075079
|
Product Name: Mitomycin C | DNA crosslinking agent (#C6486)
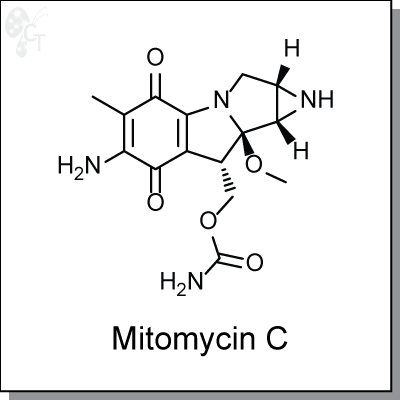
Mitomycin C is a quinone-based antitumor antibiotic that crosslinks the complementary strands of the DNA
double helix with exquisite base- and regioselectivity for the N2 of guanine. (1) It first entered clinical trials in
1958 has been employed since 1974 in combination chemotherapy treatments (2)
Mitomycin C has been shown to induce apoptosis through caspase processing. In MCF-7 breast cancer cell
lines, mitomycin C treatment led to active caspase-7, -8, and -9 processing, with minimal effect to caspase-
3. (3)
More recently, mitomycin C was observed to act irreversibly upon thioredoxin reductase (TrxR) in a time-
and concentration-dependent manner. In DTNB and insulin assays, mitomycin C inhibited TrxR at IC50s of 3
uM and 1 uM, respectively. (4)
|
Details
|
Chemical Formula:
|
|
C15H18N4O5
|
|
CAS No.:
|
|
50-07-7
|
|
Molecular weight:
|
|
334.33
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
purple solid
|
|
Chemical name:
|
|
[6-Amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazireno[2',3':3,4]pyrrolo[1,2-a]indol-8-yl]methyl carbamate
|
|
Solubility:
|
|
Up to 20 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution at -20oC
|
References
1. Tomasz, M., Mitomycin C: small, fast, and deadly (but very selective). Chem. Biol. 1995, 2, 575-579.
Pubmed ID: 9383461
2. Crooke et al., Mitomycin C: a review. Cancer Treatment Rev. 1976, 3, 121-139. Pubmed ID: 786455
3. Pirnia et al., Mitomycin C induces apoptosis and caspase-8 and -9 processing through a caspase-3 and
Fas-independent pathway. Cell Death Differentiation, 2002, 9, 905-914. Pubmed ID: 12181741
4. Paz et al., A New Mechanism of Action for the Anticancer Drug Mitomycin C: Mechanism-Based Inhibition
of Thioredoxin Reductase. Chem. Res. Toxicol. 2012, 25, 1502-1511. Pubmed ID: 786455
|
Product Name: MK-0518 (Raltegravir) | HIV-1 integrase inhibitor (#6051-5)
Raltegravir (Isentress)is an first-in-class, orally-available, oxadiazole-based inhibitor of HIV-1 integrase,
inhibiting strand transfer in vitro with an IC50 of 2-7 nM. [1] It is >1000-fold more selective for HIV-1 integrase
when compared to other phosphoryltransferases, such as the polymerase and RNase H activities of HIV-1
reverse transcriptase and the human polymerases alpha, beta, and gamma.
Raltegravir has a IC95 in human T lymphoid cell cultures of 31 nM and was also active against HIV-2 when
tested in CEMx174 cells, with an IC95 of 6nM. [2]
Raltegravir has been shown to be a potent and selective against Xenotropic murine leukemia-related
retrovirus (XMRV) at submicromolar concentrations in MCF-7 breast cancer (EC50 = 5 nM, EC90 = 3.5 uM)
and LNCaP prostate cancer (EC50 = 30 nM, EC90 0.46 um) cell lines. [3]
|
Details
|
Chemical Formula:
|
|
C20H21FN6O5
|
|
CAS No.:
|
|
871038-72-1
|
|
Molecular weight:
|
|
444.42
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical name:
|
|
N-(4-fluorobenzyl)-5-hydroxy-1-methyl-2-(2-(2-methyl-1,3,4-oxadiazole-5-carboxamido)propan-2-yl)-6-oxo-1,6-dihydropyrimidine-4-carboxamide
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
Raltegravir, MK-0518, MK0518, Isentress
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution at -20oC
|
References
1. Temesgen et al., Raltegravir: first in class HIV integrase inhibitor. Ther. Clin. Risk Management, 2008, 4(2),
493-500. Pubmed ID: 18728839
2. Hicks et al., Raltegravir: the first HIV type 1 integrase inhibitor. Clin. Infect. Dis. 2009, 48(7), 931-939.
Pubmed ID: 19231980
3. Singh et al., Raltegravir is a potent inhibitor of XMRV, a virus implicated in prostate cancer and chronic
fatigue syndrome. PloS ONE 2010, 4(4), e9948. Pubmed ID: 20376347
|
Product Name: Pirarubicin | DNA intercalator (#C7461-5)
Pirarubicin is an anthracycline drug used to treat various types of cancer. It intercalates between base pairs
and interacts with topoisomerase II, therefore inhibiting DNA replication and repair and RNA synthesis.
Compared with its analog Adriamycin (Doxorubicin), Pirarubicin is less cardiotoxic than doxorubicin and
exhibits activity against some doxorubicin-resistant cell lines. (1,2)
|
Details
|
Chemical Formula:
|
|
C32H37NO12
|
|
CAS No.:
|
|
72496-41-4
|
|
Molecular weight:
|
|
627.64
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
orange red
|
|
Chemical name:
|
|
(8S,10S)-10-(((2R,4S,5S,6S)-4-amino-6-methyl-5-(((R)-tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or DMSO solution at -20oC
|
References
1. Hirano S et al., Comparison of cardiotoxicity of pirarubicin, epirubicin and doxorubicin in the rat. Drugs Exp
Clin Res. 1994, 20(4):153-60. Pubmed ID: 7813387
2. Schott B and Robert J., Comparative cytotoxicity, DNA synthesis inhibition and drug incorporation of eight
anthracyclines in a model of doxorubicin-sensitive and -resistant rat glioblastoma cells. Biochem
Pharmacol. 1989, 38(1):167-72. Pubmed ID: 2910297
|
|




