Updated exosome products including HEK293 cell line
본문
|
Updated cell lines for exosome production includes also HEK293 cell line (Human Embryonic Kidney cells 293)
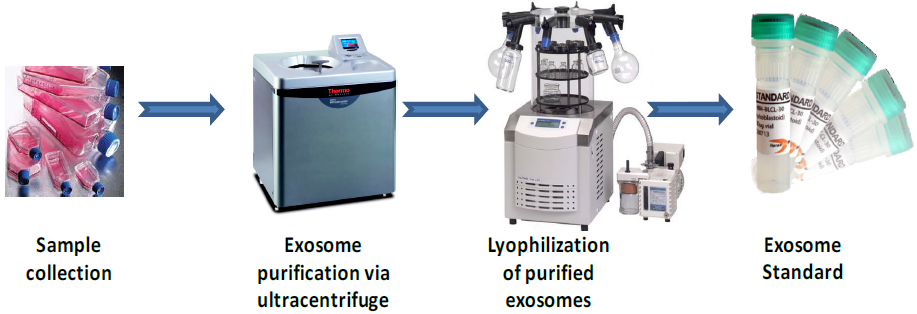
Lyophilized and purified exosomes from cell culture medium
• PC3 cell line (human prostate adenocarcinoma grade IV) • BPH-1 cell line (human being prostatic hyperplasia) • DAUDI cell line (human burkitt lymphoma) • A549 cell line (lung carcinoma) • K-562 cell line (pleural effusion, leukemia chronic myelogenous) • B16F10 mouse cell line (mouse melanoma cell line) • HEK293 cell line (human embryonic kidney)
Lyophilized Exosome Standards Lyophilization is the ideal technique for preserving the long-term stability of exosomes at 4℃. Lyophilized exosomes can be used as control standards for multiple applications including FACS, WB, ELISA and as calibration standards for quantitation of exosome-derived markers from biological samples. Lyophilized exosomes are easy to ship and store, and are stable for 12 months at 4℃. Purified and lyophilized exosomes are obtained from a variety of biological sources (cell culture supernatant, human plasma, serum, urine and saliva). Exosomes are purified following a combination of ultracentrifugation and microfiltration steps. Exosomes are subsequently quantified and validated for overall protein contentand particle number by NTA (Nanoparticles Tracking Analysis) with NanoSight LM10. Lyophilization does not affect the stability of purified exosomes and the expression levels of their exosome markers (proteins and nucleic acids).
Applications • Assay calibration • Control (spike-in) for exosome quantification • Protein marker analysis with different techinques • Extraction and analysis of exosome nucleic acid • Standardized positive controls for immunocapture performance evaluation • Flow cytometry • Electron microscopy
Advantages • Higher purity and better performance than competitor • Easy to reconstitute • Long term storage (36 months in lyophilized form, stored at 4℃) • Available from different human tumor cell lines • Available from human healthy donors body fluids (Plasma, Serum, Urine & Saliva)
Lyophilization is the ideal method for preserving exosome stability
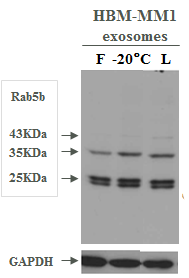
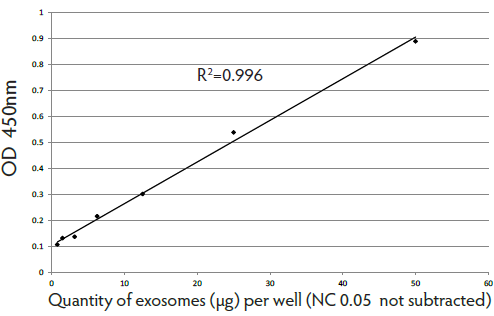
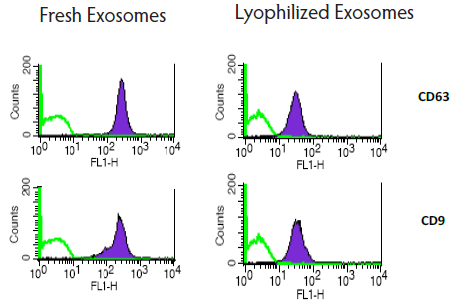
Expression of exosomal markers was assayed with different techniques (WB, FACS, ELISA) and nanovesicle count was measured with NTA. Lyophilization did not substantially affect exosome count or biomarker expression compared to other storage methods (Fig 1, 2, 3, 4).
Fig. 1. Western Blot comparison of exosomal markers on fresh (F), frozen (-20℃) and lyophilized exosomes (L)
Fig. 2. Titration of exosome standards purified from cell culture supernatant with CD63 detection.
Fig. 3. Comparison of exosomal markers on fresh and lyophilized exosomes'
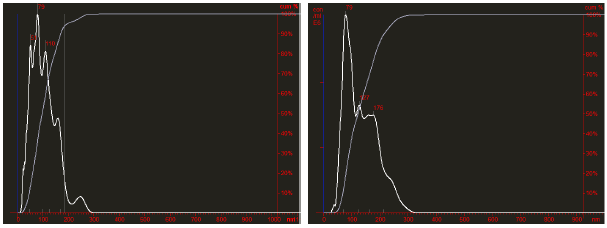
Fig. 4. Comparative Nanosight analysis of freshly purified (right panel) and lyophilized plasma exosomes (left panel).
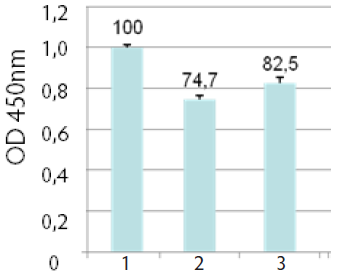
Comparing different storage methods of exosome standards (fresh vs. frozen vs lyophilized) with an anti- CD81 ELISA assay, the loss of signal compared to fresh material is minimal when using lyophilized exosomes (CV - 15%) (Fig 5).
Fig. 5. ExoTEST™ comparative detection of CD81 on HMB-MM1 derived exosomes: #1. Fresh exosomes #2. Frozen exosomes (-20℃) #3. Lyophilized exosomes
HBM Exosome Standards guarantee higher purity and performances over competitors
HBM Exosome Standard from human serum (HBM-PES) were compared to human serum exosome standard from a Competitor.
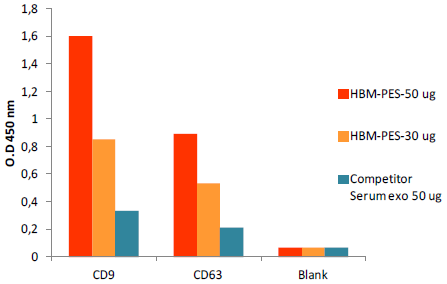
Fig. 6. ELISA quantification of HBM Exosome Standard vs Competitor’s standard (human serum) for exosomal markers CD9 and CD63
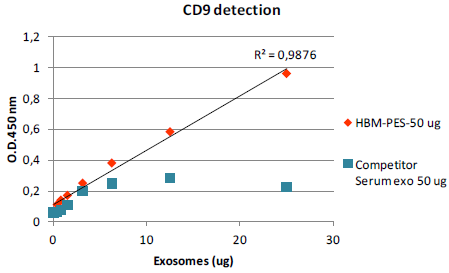
Fig. 7. Standard curve for exosome quantification. HBM Exosome Standard vs. Competitor’s standard
Exosomal markers CD9 and CD63 from 50 μg of both serum exosome standards were detected using an ELISA assay (fig 6). HBM Standard (50μg) generated the highest signal, whereas the signal from Competitor Standard (50 μg) was lower than 30 μg of HBM Standard. In a second test, 50 μg of HBM and competitor’s Standards were serially diluted to design a standard curve for exosome quantification (fig 7). The standard curve generated with HBM Standard was linear (R2 = 0,9876) within the concentration range and suitable for exosome quantification.
|
Ordering informations
- Lyophilized Exosomes from Human Biofluids (healthy donors)
|
Catalog No. |
Product Name |
Size |
|
HBM-PEP |
Human Plasma from pools of healthy donors |
30 μg x 2 vials 30 μg x 4 vials 30 μg x 6 vials 100 μg x 2 vials 100 μg x 4 vials 100 μg x 6 vials |
|
HBM-PES |
Human Serum from pools of healthy donors |
|
|
HBM-PEU |
Human Urine from pools of healthy donors |
|
|
HBM-PESL |
Human Saliva from pools of healthy donors |
- Lyophilized Exosomes from Cell Supernatants (human tumor cell lines)
|
Catalog No. |
Product Name |
Size |
|
HBM-COLO |
Human Colon Carcinoma cell lines |
30 μg x 2 vials 30 μg x 4 vials 30 μg x 6 vials 100 μg x 2 vials 100 μg x 4 vials 100 μg x 6 vials |
|
HBM-MM1 |
Human Melanoma cell lines |
|
|
HBM-BLCL21 |
EBV transformed lymphoblastoid B cell line |
|
|
HBM-U87 |
Human glioblastoma-astrocytoma cell line |
|
|
HBM-HCT |
Human colon carcinoma cell line |
|
|
HBM-SK |
Human neuroblastoma cell line |
|
|
More than 100 additional cell lines are available for exosome purification or cell lysates on request. |
||
* Lyophilized Exosome Standards are sold in vials containing 100 μg or 30 μg of total protein
(100 μg: Particles/ml > 1x10^10. 30 μg: Particles/ml > 1x10^8)
▣ 관련 페이지 ; HansaBioMed
- 이전글Primary human glioblastoma cells 18.01.06
- 다음글Minute™ Hi-efficiency Saliva Exosome Isolation Kit 17.12.13
댓글목록
등록된 댓글이 없습니다.